New research suggests that certain dopamine receptors in the brain and body are more complex and dynamic than previously thought, existing in diverse inactive states that only allow the binding of specific drugs. These findings bring a level of detail to our understanding of dopamine receptors that is critical to more efficient development of medications to treat addiction and other disorders. The computational analysis was coordinated by NIDA’s Intramural Research Program (IRP).
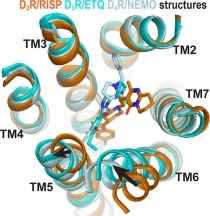
Almost a third of prescribed drugs work by acting on a group of proteins known as G-protein coupled receptors (GPCRs), which help to transmit messages across the cell’s outer barrier. The neurotransmitter dopamine, for example, acts by attaching to dopamine receptors, a sub-family of GPCRs. GPCRs have a unique shape-shifting talent; with different activating molecules, they can adopt different shapes. This in turn creates a molecular cascade that changes the three-dimensional structure (or conformation) of the receptor from an inactive to active state. Some GPCRs can have different inactive structures, but it has been unclear if this was the case for the dopamine D2 and D3 receptors, which can play a role in the development of addiction and other mental health conditions. Mapping out these inactive conformations of receptors is important to drug discovery.
Medications used to treat substance use disorders can be: agonists, which activate the receptors they bind to; partial agonists, which active the receptors less strongly than full agonists; or antagonists, which block the activation of those receptors. In this study, investigators simulated and compared the interaction of D2 receptors with the dopamine antagonists risperidone and eticlopride and found each drug led to a different conformation. In addition, D2 and D3 showed a very similar conformation when attached to eticlopride, as the two drugs bound to the inactive receptors in overlapping but different locations. Overall, these computational findings contribute to a more refined understanding of the complex conformations of the dynamic and diverse GPCRs.
The work was done by scientists at NIDA’s Intramural Research Program in collaboration with the University of Nottingham, England and Columbia University in New York City.
Study:
- Lane, Robert J; Abramyan, Ara M; Adhikari, Pramisha; Keen, Alastair C; Lee, Kuo-Hao; Sanchez, Julie; Verma, Ravi Kumar; Lim, Herman D; Yano, Hideaki; Javitch, Jonathan A; Shi, Lei. Distinct inactive conformations of the dopamine D2 and D3 receptors correspond to different extents of inverse agonism. eLife.