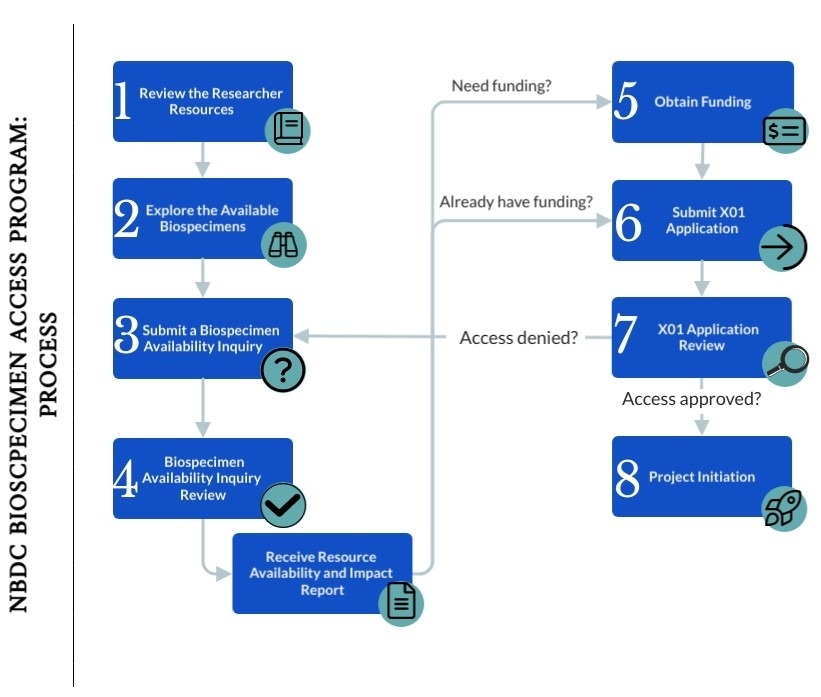
The NIH Brain Development Cohorts (NBDC) Biospecimen Access Program provides the research community access to biospecimens collected from the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®) participants through an X01 resource access mechanism.
- Introduction
What is the ABCD Study?
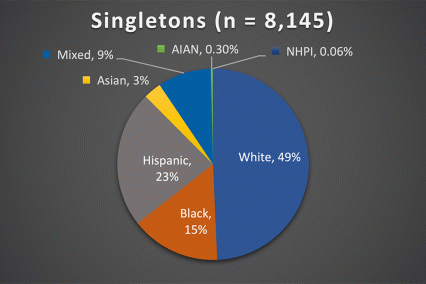
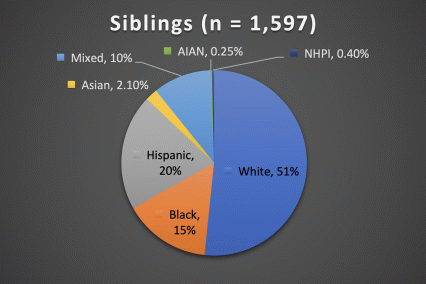
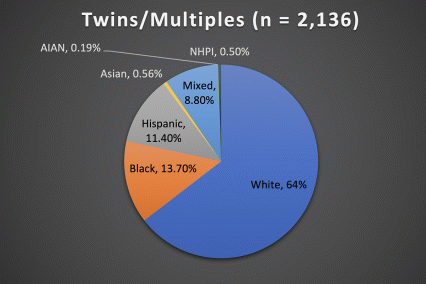
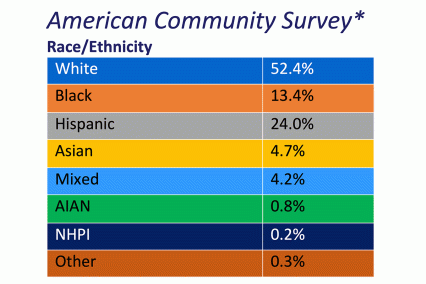
The ABCD Study is the largest longitudinal study of brain development and child health in the U.S., collecting data from nearly 12,000 youth across the country beginning when they were 9-10 years old and continuing for a decade. The ABCD Study cohort approximates the U.S. population on several demographic factors (Figure 1. Baseline enrollment: n=11,878 participants; 48% female, 52% male)
Figure 1. Race/Ethnicity of Participants in the ABCD Study at Baseline
*The American Community Survey is a demographics survey conducted by the U.S. Census Bureau. Note that the ABCD Study participant race/ethnicity distribution closely resembles the American Community Survey distribution.
The ABCD Study collects data related to physical and mental health, culture and environment, substance use, and neurocognitive function, as well as bio-assays for hormones, cardiovascular and hematologic health, genetics, environmental toxins, and substance use analysis, and multimodal brain imaging to track changes in brain structure and function as participants transition from childhood through adolescence into adulthood. See the ABCD Study site for detailed protocols.
Accessing shared data from the ABCD Study
Data from the ABCD Study is currently being shared through the NIMH Data Archive. Please visit the ABCD Data Sharing page for more information.
Available Resources
Stored biospecimens from the ABCD Study include:
Biospecimen and collection frequency table Biospecimen Collection Frequency Oral Fluid Annually Shed Teeth Baseline/as shed DNA-Saliva Primarily baseline Whole Blood and DNA-blood Twins - Every other year beginning at baseline
Remaining cohort - Every other year beginning at 2-year follow-upSerum Every other year beginning at 2-year follow-up Biospecimen collection was interrupted for the 2-, 3-, and 4-year follow-up visits and availability is variable across the cohort due to the COVID-19 pandemic. Researchers can explore the number of participants with available biospecimens via the NBDC Portal Biospecimen Explorer.
- Applying for Access to Biospecimens
Follow the steps below to plan, prepare, and submit your X01 application:
- Review the Researcher Resources
The NBDC Portal Researcher Guide has detailed information on available biospecimens and relevant collection, processing, and storage details to aid researchers in determining whether the ABCD Study samples will meet their study needs. - Explore the Available Biospecimens
Researchers can explore the number of participants with available biospecimens via the NBDC Portal Biospecimen Explorer and filter for the specimen type, visit timepoint, and demographic variables of interest. - Submit a Biospecimen Availability Inquiry
Once you have created an NBDC portal account, you can apply your Biospecimen Explorer search to create a Biospecimen Availability Inquiry. Applicants should submit a Biospecimen Availability Inquiry via the NBDC Portal at least four weeks in advance of their X01 submission (if funding is already obtained) or funding application submission (if funding is not yet obtained), to determine whether there is sufficient quantity of the sample required for the proposed study, the impact it will have on the resource, and whether the proposed use of samples is consistent with goals of the program. Biospecimen Availability Inquiries may be submitted on a rolling schedule.
Applicants who are attempting to find biospecimens for specific participants based on other available ABCD Study data should contact the NBDC Biospecimen team before submitting their inquiry. - Biospecimen Availability Inquiry Review
Upon receipt of your Biospecimen Availability Inquiry, the NBDC Biospecimen Team – made up of NIH staff and biorepository experts – will determine whether your requested samples are available or recommend adjustments to your inquiry as needed. You will be provided with a Resource Availability and Impact Report that should be included when applying for funding (if not already obtained) and with your X01 application. - Apply for funding (if not yet obtained)
Applicants must provide evidence of funding to support research proposed using ABCD Study biospecimens with their X01 application (e.g., Notice of Grant Award). This funding must include costs of conducting the research and costs for shipment of biospecimens from the repository to the investigator. If you do not yet have funding to conduct the proposed research, you will need to secure it before applying for the X01.
Active funding opportunities to consider:- RFA-DA-22-037 - Accelerating the Pace of Drug Abuse Research Using Existing Data (R01 Clinical Trial Optional)
- RFA-DA-22-038 - Accelerating the Pace of Drug Abuse Research Using Existing Data (R21 Clinical Trial Optional)
- PAR-22-137 - Accelerating the Pace of Child Health Research Using Existing Data from the Adolescent Brain Cognitive Development (ABCD) Study (R01-Clinical Trial Not Allowed)
- PAR-22-138 - Accelerating the Pace of Child Health Research Using Existing Data from the Adolescent Brain Cognitive Development (ABCD) Study (R21-Clinical Trial Not Allowed)
- The Human Health Exposure Analysis Resource (HHEAR) laboratories for chemical analyses of banked biological samples to support characterization of environmental exposure.
- NIH parent announcements
- X01 Application Submission
- Review the NIH Developmental Studies Biospecimen Application Submission Schedule in PAR-23-229 – NIH Brain Development Cohorts (NBDC) Biospecimens Access (X01) Notice of Funding Opportunity (NOFO) and plan your submission accordingly.
- Use the Application Guide to prepare your application. When you are ready to submit an application, go to the link in the NBDC X01 NOFO to complete the SF424 Research and Related (R&R) forms and submit them through Grants.gov. Applicants will be required to:
- Include the Resource Availability and Impact Report as a PDF file labeled “Resource Availability and Impact Report” in Other Attachments.
- Submit proof of funding to carry out the research, including costs for shipment of samples to the investigator, as a PDF file labeled “Funding Source” in Other Attachments. Applicants must identify their funding source and include relevant documentation (e.g., Notice of Award).
- In addition to the X01 submission, applicants must submit the following in the NBDC Portal
- If the scientific merit of the proposed research was evaluated by the funding source, provide documentation of the evaluation (e.g. Summary Statement) .
- Applicants are expected to address all elements from the Data Management and Sharing Plan, including those specific to genomic data sharing if genetic analyses are proposed, as required by the NBDC Sample and Data Use Agreement.
- Application Review
- Upon receipt of your application in Grants.gov, the Center for Scientific Review (CSR), NIH, will evaluate it for completeness and compliance with application instructions. When the administrative review is complete, CSR will forward your application to the NBDC Biospecimen Access Committee (BAC). The NBDC BAC is composed of federal staff and scientists with appropriate expertise.
- The NBDC BAC will review your application using the review criteria listed in the NBDC Biospecimens Access X01 NOFO. The NBDC BAC will provide a summary of their review to the NBDC Biospecimen Team.
- Resource Access notifications will be made within three months of X01 review. You will receive a final decision by email notification. Written critiques will be accessed through the NBDC portal.
- Review the Researcher Resources
- Project Initiation
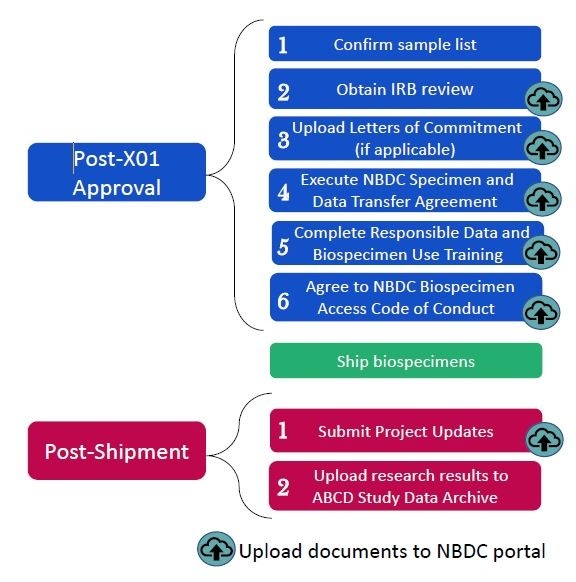
Your resource access notification will include instructions on how to initiate post-approval activities, including biospecimen shipment, and reporting requirements.
Post-approval activities
These activities must be completed by the applicant within three months of the date on the approval notification. Documents should be uploaded to the NBDC portal:
- Confirm sample list: The NBDC Biospecimen Team will provide a biospecimen manifest via the NBDC portal that lists detailed information on the samples that will be shipped to you. Confirm the list of samples.
- Institutional Review Board (IRB) letter: Submit a letter from your institution’s IRB documenting approval or exemption of your proposed project.
- Letters of Commitment: If using biospecimens from other studies, provide letters of commitment from collaborators.
- NBDC Specimen and Data Transfer Agreement (SDTA): Execute the NBDC SDTA (also known as a Material Transfer Agreement (MTA)) covering the transfer of biospecimens and associated data from the ABCD Study biorepository(ies) to your designated laboratory. The NBDC Biospecimen Team provides a standard form via the NBDC portal.
- Complete the NBDC Responsible Data and Biospecimen Use Training, and upload the certification of completion to the NBDC portal.
- Agree to the NBDC Biospecimen Access Code of Conduct.
Post-shipment activities
- Submit Project Updates
Annual Project Updates: You will be expected to submit annual project updates starting one year after your biospecimen samples are shipped, which will be reviewed by the NBDC BAC.
The project update should include:
- Timeline/progress on biospecimen analyses;
- Progress on data analysis;
- Publications/presentations resulting from the proposed analyses;
- Changes to funding or key personnel;
- Demonstration of data sharing via the ABCD Study data archive if analyses are complete.
Final Project Update: Your final update is due six months after you complete your biospecimen analyses.
The project update should include:
- Status of residual biospecimens and documentation of completion of the disposal/return (see award notification);
- Demonstration of data sharing via the ABCD Study data archive;
- Resulting manuscript citations.
- Upload research results to the ABCD Study data archive
- After completion of the proposed analysis, all research results must be de-identified, including non-study data that may be included in the analysis, and deposited in the ABCD Study data archive. When submitting data, recipients must link their analyses to the relevant ABCD Study collection and participants.
- Recipients will be required to agree to the ABCD Study data archives' Data Use Certification in order to submit research data.
- Acknowledgements
- Recipients must use the Research Resource ID associated with the ABCD Study, SCR_015769, when referencing ABCD Study materials in data presentations and published manuscripts.
- Recipients must use appropriate acknowledgement language to reference the ABCD Study as the source of the samples:
Biospecimens {and/or Derivatives} were provided by the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), an initiative of the National Institutes of Health, through NIDA Contract # 75N95021D00023 and an MOU with ACTRI. - Recipients must use appropriate acknowledgement language to reference the ABCD Study as the source of the original data made available through the NIMH Data Archive: Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit nearly 12,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA050987, U01DA050988, U01DA050989, U01DA051016, U01DA051018, U01DA051037, U01DA051038, U01DA051039, U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. The ABCD Study website also has a listing of participating sites and a complete listing of the study investigators. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. (Add the following sentence for a report that uses data from a versioned release) The ABCD data repository grows and changes over time. The ABCD data used in this report came from [NIMH Data Archive Digital Object Identifier (DOI)]. DOIs can be found at [DOI URL]. (Add the following sentence for a report that uses data from the fast track release) The ABCD data repository grows and changes over time. The ABCD data used in this report came from the fast track data release. The raw data are available at [NIMH Data Archive Digital Object Identifier (DOI)].
- Frequently Asked Questions
Application details
- Do I have to have funding before I apply?
Yes. You will be required to provide proof of funding, including funding source and related documentation (e.g., Notice of Award), in your X01 application to cover all associated costs of conducting the research and shipping the biospecimens to the investigator.
If the scientific merit of the proposed research was evaluated by the funding source, provide documentation of the evaluation (e.g. Summary Statement) in the NBDC portal. - Who can apply for access?
Please see the NBCD X01 NOFO: PAR-23-229 for information about eligibility. Both foreign (non-U.S.) and domestic (U.S. based) investigators from eligible institutions as specified in the NOFO may apply. Investigators with proof of funding for studies consistent with research priorities for child or adolescent health and development will be given priority. - Can a non-U.S. based investigator apply for access to samples from these studies?
Yes, samples are available to U.S. – and non- U.S. - based investigators. However, investigators from foreign institutions will need to pay for costs associated with shipping samples outside the United States. If the shipment is intended for a foreign country, the researcher is responsible for obtaining the required documents for entry of biohazardous material. - Can I make changes to the proposed study details?
Please contact the study team if changes are required after X01 application submission. Substantial changes may require an addendum or resubmission of the X01 application. - Can I submit more than one application at a time?
You may submit more than one application at a time if the study hypotheses and aims are different. - Can I resubmit if my application is rejected?
If your application is not approved, you should contact the NBDC Biospecimen Team at devstudybiosamples@nida.nih.gov to discuss your options for re-submission. You may be asked to revise and resubmit both the Biospecimen Availability Inquiry and X01 application or to revise and resubmit only the application.
Review
- Who will review my Biospecimen Availability Inquiry?
Your Biospecimen Availability Inquiry will be reviewed by the NBDC Biospecimen Team. The NBDC Biospecimen Team includes members from the biorepositories and NIH staff. - What is the timeframe for review of a Biospecimen Availability Inquiry?
Biospecimen Availability Inquiry review takes approximately three months. The review process will include checking for availability of requested samples, impact to the resource, and consistency with the goals of the program. You will be provided with a Resource Availability and Impact Report at the end of the review period. - Who will review my X01 application?
Your X01 application will be reviewed by the NBDC Biospecimen Access Committee (BAC). NBDC BAC members are federal employees familiar with the ABCD Study, who have expertise in biospecimens and laboratory science, as well as in child and adolescent development. The NBDC BAC organizers may call upon experts in other research areas to help with specific reviews. - What is the timeframe for review of an application?
Application review takes approximately two months, after which you will be emailed a notification of the decision. - Do I need IRB approval prior to submitting my Biospecimen Availability Inquiry?
You do not need IRB approval prior to submitting a Biospecimen Availability Inquiry or an X01 application. However, you will be required to submit proof of IRB approval or exemption for your proposed use of samples after your application is approved and before your samples will be shipped.
Post-approval questions
- After my application is approved, how long until I receive my shipment?
Upon approval, you must submit additional documents before samples are shipped (see post-approval activities above). You have up to three months after the date on your approval notification to send these documents. Upon receipt, samples will be prepared and shipped. - Do I need to pay for retrieving or shipping the ABCD Study samples?
Investigators will pay for shipping expenses, but there is no cost for the samples themselves. - Am I required to submit or share the results from my study?
You are required to share research results and data with the ABCD Study data archive, linking your analyses to the ABCD Study collection and participants, consistent with the NIH Data Sharing Policy and the policies set forth by the project funding agencies. - Are there limitations to the data sharing process or to the types of data that can be shared?
All data must be de-identified before uploading to the ABCD Study data archive. You may not transfer data, with or without charge, to any other entity or any individual. This applies to all versions of ABCD Study data (including any individual-level data derived from the original data).
- Do I have to have funding before I apply?
Contact team
For inquiries regarding the NIH Brain Development Cohorts Biospecimen resource and procedures for access, contact the NBDC Biospecimen Team.