Abundant evidence shows that methadone, buprenorphine, and naltrexone all reduce opioid use and opioid use disorder-related symptoms, and they reduce the risk of infectious disease transmission as well as criminal behavior associated with drug use.15 These medications also increase the likelihood that a person will remain in treatment, which itself is associated with lower risk of overdose mortality, reduced risk of HIV and HCV transmission, reduced criminal justice involvement, and greater likelihood of employment.15
Methadone
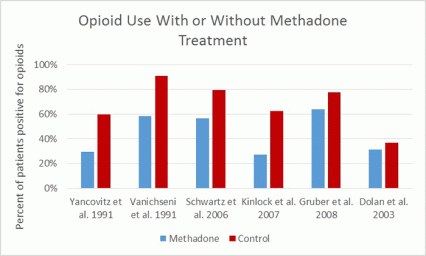
Methadone is the medication with the longest history of use for opioid use disorder treatment, having been used since 1947. A large number of studies (some of which are summarized in the graph below) support methadone's effectiveness at reducing opioid use. A comprehensive Cochrane review in 2009 compared methadone-based treatment (methadone plus psychosocial treatment) to placebo with psychosocial treatment and found that methadone treatment was effective in reducing opioid use, opioid use-associated transmission of infectious disease, and crime.12,16–20 Patients on methadone had 33 percent fewer opioid-positive drug tests and were 4.44 times more likely to stay in treatment compared to controls.12 Methadone treatment significantly improves outcomes, even when provided in the absence of regular counseling services;18,19,21 long-term (beyond 6 months) outcomes are better in groups receiving methadone, regardless of the frequency of counseling received.22,23
Buprenorphine
Buprenorphine, which was first approved in 2002, is currently available in two forms: alone (Probuphine®, Sublocade™, Bunavail®) and in combination with the opioid receptor antagonist naloxone (Suboxone®, Zubsolv®). Both formulations of buprenorphine are effective for the treatment of opioid use disorders, though some studies have shown high relapse rates among patients tapered off of buprenorphine compared to patients maintained on the drug for a longer period of time.24
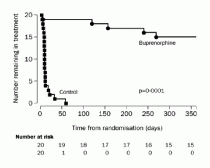
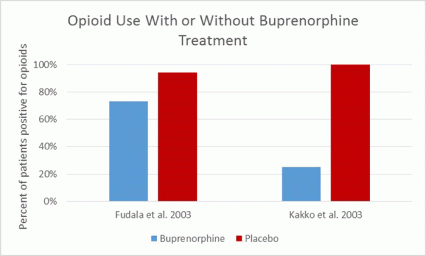
A Swedish study compared patients maintained on 16 mg of buprenorphine daily to a control group that received buprenorphine for detoxification (6 days) followed by placebo.25 All patients received psychosocial supports. In this study, the treatment failure rate for placebo was 100 percent vs. 25 percent for buprenorphine. More than two opioid-positive urine tests within 3 months resulted in cessation of treatment, so treatment retention was closely related to relapse. Of patients not retained in treatment, there was a 20 percent mortality rate.
Meta-analysis determined that patients on doses of buprenorphine of 16 mg per day or more were 1.82 times more likely to stay in treatment than placebo-treated patients, and buprenorphine decreased the number of opioid-positive drug tests by 14.2 percent (the standardized mean difference was -1.17).13,25,26
To be effective, buprenorphine must be given at a sufficiently high dose (generally, 16 mg per day or more). Some treatment providers wary of using opioids have prescribed lower doses for short treatment durations, leading to failure of buprenorphine treatment and the mistaken conclusion that the medication is ineffective.13,27
Methadone and Buprenorphine Compared
Methadone and buprenorphine are equally effective at reducing opioid use. A comprehensive Cochrane review comparing buprenorphine, methadone, and placebo found no differences in opioid-positive drug tests or self-reported heroin use when treating with methadone or buprenorphine at medium-to-high doses.13
Notably, flexible dose regimens of buprenorphine and doses of buprenorphine of 6 mg or below are less effective than methadone at keeping patients in treatment, highlighting the need for delivery of evidence-based dosing regimens of these medications.13
Naltrexone
Naltrexone was initially approved for the treatment of opioid use disorder in a daily pill form. It does not produce tolerance or withdrawal. Poor treatment adherence has primarily limited the real-world effectiveness of this formulation.28 As a result, there is insufficient evidence that oral naltrexone is an effective treatment for opioid use disorder.29 Extended-release injectable naltrexone (XR-NTX) is administered once monthly, which removes the need for daily dosing. While this formulation is the newest form of medication for opioid use disorder, evidence to date suggests that it is effective.28,30
The double-blind, placebo-controlled trial that was most influential in getting XR-NTX approved by the FDA in 2010 for opioid use disorder treatment showed that XR-NTX significantly increased opioid abstinence. The XR-NTX group had 90 percent confirmed abstinent weeks compared to 35 percent in the placebo group. Treatment retention was also higher in the XR-NTX group (58 percent vs. 42 percent), while subjective drug craving and relapse were both decreased (0.8 percent vs. 13.7 percent).31 Improvement in the XR-NTX group was sustained throughout an open label period out to 76 weeks.32 These data were collected in Russia, and additional studies are required to determine if effectiveness will be similar in the United States.33
Buprenorphine and Naltrexone Compared
A NIDA study showed that once treatment is initiated, a buprenorphine/naloxone combination and an extended release naltrexone formulation are similarly effective in treating opioid use disorder. Because naltrexone requires full detoxification, initiating treatment among active opioid users was more difficult with this medication. However, once detoxification was complete, the naltrexone formulation had a similar effectiveness as the buprenorphine/naloxone combination.