In 2024, NIDA celebrated its 50th anniversary. On May 14, 1974, an act of Congress established the National Institute on Drug Abuse (NIDA), and since then NIDA has led the world in funding and conducting research on drug use and addiction. NIDA research has been instrumental in shifting the mindset of medicine and the public from viewing addiction as deviance toward understanding it as a health condition that is preventable, treatable, and deserving of compassion and support.
Related Stories
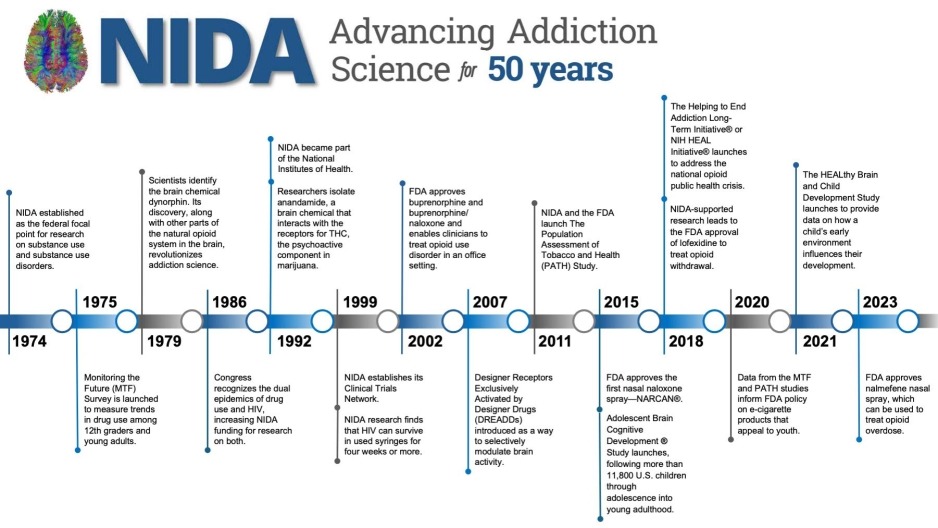
Timeline of Notable Achievements
- Long Description for Timeline graphic
- 1974: NIDA established as the federal focal point for research on substance use and substance use disorders.
- 1975: Monitoring the Future (MTF) Survey is launched to measure trends in drug use among 12th graders and young adults.
- 1979: Scientists identify the brain chemical dynorphin. Its discovery, along with other parts of the natural opioid system in the brain, revolutionizes addiction science.
- 1986: Congress recognizes the dual epidemics of drug use and HIV, increasing NIDA funding for research on both.
- 1992: NIDA became part of the National Institutes of Health.
Researchers isolate anandamide, a brain chemical that interacts with the receptors for THC, the psychoactive component in marijuana. - 1999: NIDA establishes its Clinical Trials Network.
NIDA research finds that HIV can survive in used syringes for four weeks or more. - 2002: FDA approves buprenorphine and buprenorphine/ naloxone and enables clinicians to treat opioid use disorder in an office setting.
- 2007: Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) introduced as a way to selectively modulate brain activity.
- 2011: NIDA and the FDA launch The Population Assessment of Tobacco and Health (PATH) Study.
- 2015: FDA approves the first nasal naloxone spray—NARCAN®.
Adolescent Brain Cognitive Development® Study launches, following more than 11,800 U.S. children through adolescence into young adulthood. - 2018: The Helping to End Addiction Long-Term Initiative® or NIH HEAL Initiative® launches to address the national opioid public health crisis.
NIDA-supported research leads to the FDA approval of lofexidine to treat opioid withdrawal. - 2020: Data from the MTF and PATH studies inform FDA policy on e-cigarette products that appeal to youth.
- 2021: The HEALthy Brain and Child Development Study launches to provide data on how a child’s early environment influences their development.
- 2023: FDA approves nalmefene nasal spray, which can be used to treat opioid overdose.