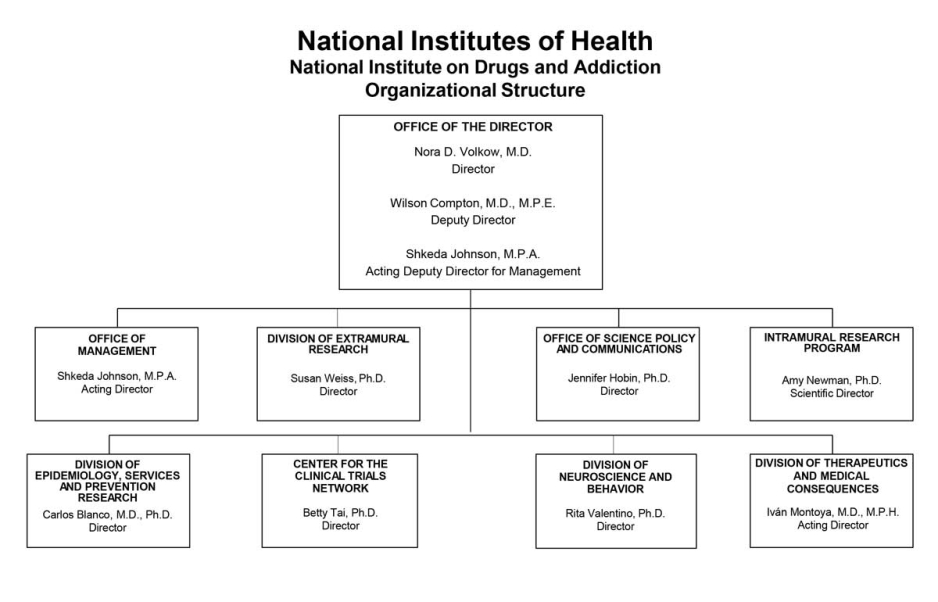
Organizational Chart
- Text Description of Organizational Chart
Office of the Director
- Nora D. Volkow, M.D.
Director - Wilson Compton, M.D., M.P.E.
Deputy Director - Shkeda Johnson, M.P.A.
Acting Deputy Director for Management
Office of Management
- Shkeda Johnson, M.P.A.
Acting Director
Division of Extramural Research
- Susan Weiss, Ph.D.
Director
Office of Science Policy and Communications
- Jennifer Hobin, Ph.D.
Director
Intramural Research Program
- Amy Newman, Ph.D.
Scientific Director
Division of Epidemiology, Services and Prevention Research
- Carlos Blanco, M.D., Ph.D.
Director
Center for the Clinical Trials Network
- Betty Tai, Ph.D.
Director
Division of Neuroscience and Behavior
- Rita Valentino, Ph.D.
Director
Division of Therapeutics and Medical Consequences
- Ivan Montoya, M.D., M.P.H.
Acting Director
- Nora D. Volkow, M.D.
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to [drug abuse, $1,662,695,000] drugs and addiction, $1,663,365,000.
Tables
- Budget Mechanism
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2022
FinalFY 2023 Enacted FY 2024
President's
BudgetFY 2024
+/-
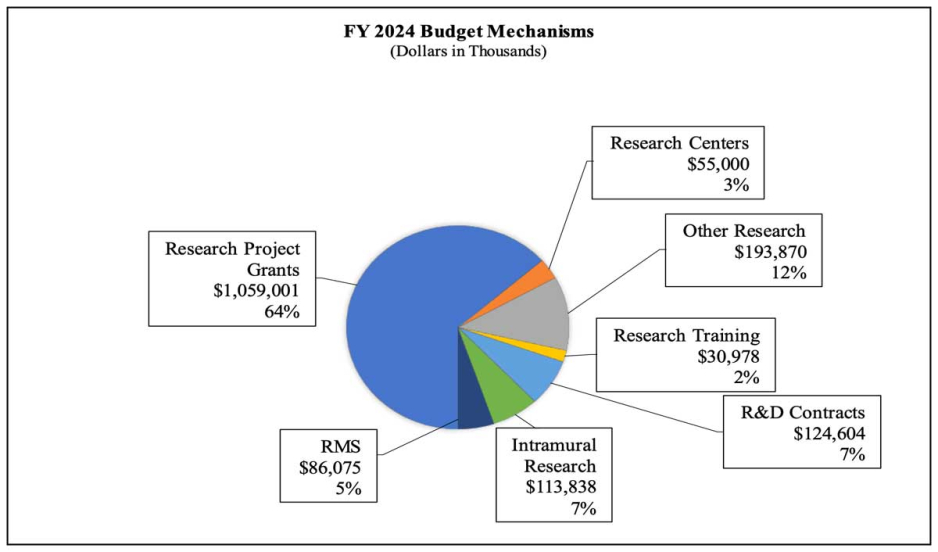
FY 2023No. Amount No. Amount No. Amount No. Amount Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Research Projects: Noncompeting 937 $645,650 1.029 $768,537 1,074 $773,763 45 $5,225 Administrative Supplements (107) $21,321 (101) $17,500 (90) $16,000 (-11) -$1,500 Competing: Renewal 29 $24,086 22 $18,000 24 $20,000 2 $2,000 New 376 $287,274 305 $193,975 265 $196,408 -40 $2,433 Supplements 0 0 0 0 0 0 0 0 Subtotal, Competing 405 $311,360 327 $211,975 289 $216,408 -38 $4,433 Subtotal, RPGs 1,342 $978,332 1,356 $988,012 1,363 $1,006,171 7 $8,158 SBIR/STTR 78 $50,641 78 $50,210 82 $52,830 4 $2,620 Research Project Grants 1,420 $1,028,973 1,434 $1,048,222 1,445 $1,059,001 11 $10,778 Research Centers: Specialized / Comprehensive 27 $59,569 27 $60,091 25 $55,000 -2 -$5,091 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 0 0 0 0 0 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 27 $59,569 27 $60,091 25 $55,000 -2 -$5,091 Other Research: Research Careers 239 $45,036 255 $48,105 261 $49,158 6 $1,053 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 27 $84,235 28 $85,920 27 $83,169 -1 -$2,751 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 $1,869 0 $2,039 0 $1,984 0 -$55 Other 77 $56,700 85 $60,960 85 $59,559 0 -$1,402 Other Research 343 $187,840 368 $197,023 373 $193,870 5 -$3,154 Total Research Grants 1,790 $1,276,382 1,829 $1,305,337 1,843 $1,307,870 14 $2,533 Individual Awards 165 $7,598 194 $9,191 196 $9,288 2 $97 Institutional Awards 351 $21,640 339 $21,162 339 $21,690 0 $527 Total Research Training 516 $29,237 533 $30,353 535 $30,978 2 $625 Research and Development Contracts 89 $109,365 103 $134,316 99 $124,604 -4 -$9,712 (SBIR/STTR) (non-add) (0) ($692) (0) ($3,532) (0) ($661) (0) (-$2,871) -Intramural Research 121 $103,867 122 $111,379 123 $113,838 1 $2,459 Res. Management & Support 275 $77,272 276 $81,890 293 $86,075 17 $4,095 Res. Management & Support (SBIR Admin) (non-add) (0) ($164) (0) ($245) (0) ($245) (0) (0) Construction 0 0 0 0 0 0 0 0 Buildings and Facilities 0 0 0 0 0 0 0 0 Total, NIDA 396 $1,596,123 398 $1,663,365 416 $1,663,365 18 $0 1 All numbers in italics and brackets are non-add entries.
- Amounts Available for Obligation
(Dollars in Thousands)1 Source of Funding FY 2022
FinalFY 2023
EnactedFY 2024
President's
BudgetAppropriation $1,595,474 $1,662,695 $1,663,365 Secretary's Transfer $0 $0 $0 OAR HIV/AIDS Transfers $649 $670 $0 Subtotal, adjusted budget authority $1,596,123 $1,663,365 $1,663,365 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $1,596,123 $1,663,365 $1,663,365 Unobligated balance lapsing -$54 0 0 Total obligations $1,596,069 $1,663,365 $1,663,365 1Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2022 - $94,083 FY 2023 - $69,000 FY 2024 - $70,000- Summary of Changes
(Dollars in Thousands) FY 2023 Enacted $1,663,365 FY 2024 President's Budget $1,663,365 Net change $0 FY 2023 Enacted FY 2024 President's Budget Built-In Change from
FY 2023 EnactedCHANGES FTEs/Budget Authority FTEs/Budget Authority FTEs/Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of January 2022 pay increase & benefits $33,036 $34,804 $367 b. January FY 2023 pay increase & benefits $33,036 $34,804 $1,268 c. Paid days adjustment $33,036 $34,804 $127 d. Differences attributable to change in FTE $33,036 $34,804 $271 e. Payment for centrally furnished services $13,231 $13,443 $212 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs$65,112 $65,590 $1,323 Subtotal $3,568 2. Research Management and Support: a. Annualization of January 2022 pay
increase & benefits$48,103 $51,402 $528 b. January FY 2023 pay increase & benefits $48,103 $51,402 $1,834 c. Paid days adjustment $48,103 $51,402 $185 d. Differences attributable to change in FTE $48,103 $51,402 $3,295 e. Payment for centrally furnished services $4,907 $4,985 $79 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs$28,971 $29,687 $606 Subtotal $6,526 Subtotal, Built-in $10,094 Summary of Changes - Continued
(Dollars in Thousands)FY 2023 Enacted FY 2024 President's Budget Program Change from
FY 2023 EnactedCHANGES No. Amount No. Amount No. Amount B. Program: 1. Research Project Grants: a. Noncompeting 1,029 $786,037 1,074 $789,763 45 $3,725 b. Competing 327 $211,975 289 $216,408 -38 $4,433 c. SBIR/STTR 78 $50,210 82 $52,830 4 $2,620 Subtotal, RPGs 1,434 $1,048,222 1,445 $1,059,001 11 $10,778 2. Research Centers 27 $60,091 25 $55,000 -2 -$5,091 3. Other Research 368 $197,023 373 $193,870 5 -$3,154 4. Research Training 533 $30,353 535 $30,978 2 $625 5. Research and development contracts 103 $134,316 99 $124,604 -4 -$9,712 Subtotal, Extramural $1,470,006 $1,463,452 -$6,554 FTEs FTEs FTEs 6. Intramural Research 122 $111,379 123 $113,838 1 -$1,109 7. Research Management and Support 276 $81,980 293 $86,075 17 -$2,431 8. Construction 0 0 0 9. Buildings and Facilities 0 0 0 Subtotal, program 398 $1,663,365 416 $1,663,365 18 -$10,094 Total built-in and program changes $0 - Authorizing Legislation
PHS Act/Other Citation 2023 Amount Author-
izedFY 2023 Enacted 2024 Amount Author
-izedFY 2024 President's Budget Research and Investigation Section 301 42§241 Indefinite $1,633,365,000 Indefinite $1,633,365,000 National Institute on Drugs and Addiction Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,633,365,000 $1,633,365,000 - Appropriations History
Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2015 $1,023,268,000 $1,028,614,000 Rescission $0 2016 $1,047,397,000 $1,050,875,000 $1,069,086,000 $1,077,488,000 Rescission $0 20171 $1,050,550,000 $1,107,700,000 $1,103,032,000 1,090,853,000 Rescission $0 2018 $864,998,000 $1,107,497,000 $1,113,442,000 $1,383,603,000 Rescission $0 2019 $1,137,403,000 $1,400,126,000 $1,420,591,000 $1,419,844,000 Rescission $0 2020 $1,296,379,000 $1,489,237,000 $1,490,498,000 $1,462,016,000 Rescission $0 2021 $1,431,770,000 $1,476,590,00 $1,505,192,000 $1,479,660,000 Rescission $0 2022 $1,852,503,000 $1,860,329,000 $1,832,906,000 $1,595,474,000 Rescission $0 2023 $1,843,326,000 $1,712,832,000 $1,684,230,000 $1,662,695,000 Rescission $0 2024 $1,663,365,000 1Budget Estimate to Congress includes mandatory financing.
- Budget Authority by Activity
(Dollars in thousands)1 Extramural Research FY 2022
FinalFY 2023 Enacted FY 2024
President's
BudgetFY 2024
+/-
FY 2023 EnactedFTE Amount FTE Amount FTE Amount FTE Amount Detail: Division of Therapeutics and Medical Consequences $124,555 $129,897 $129,312 -$585 Division of Neuroscience and Behavior $514,315 $536,374 $533,959 -$2,415 Division of Epidemiology, Services and Prevention Research $358,859 $374,251 $372,566 -$1,685 Center for the Clinical Trials Network $39,317 $41,003 $40,818 -$185 Office of Translational Initiatives and Program Innovations $41,155 $42,921 $42,727 -$193 HEAL Initiative2 $336,784 $345,560 $344,069 -$1,491 Subtotal, Extramural $1,414,985 $1,470,006 $1,463,452 -$6,554 Intramural Research 121 $103,867 122 $111,379 123 $113,838 1 $2,459 Research Management & Support 275 $77,272 276 $81,980 293 $86,075 17 $4,095 TOTAL 396 $1,596,123 398 $1,663,365 398 $1,663,365 18 $0 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Total for HEAL Initiative including RMS is (in thousands) $345,295 in FY 2022, $355,295 in FY 2023, and $355,295 in FY 2024.- Budget Authority by Object Class
(Dollars in Thousands)1 FY 2023 Enacteed FY 2024 President's Budget FY 2024 +/- FY 2023 OBJECT CLASSES FY 2023 Enacted FY 2024 President's Budget FY 2024 +/- FY 2023 Total compensable workyears: Full-time equivalent 398 416 18 Full-time equivalent of overtime and holiday hours 0 0 0 Average ES salary $214 $225 $11 Average GM/GS grade 13.0 13.0 0.0 Average GM/GS salary $139 $145 $7 Average salary, Commissioned Corps (42 U.S.C. 207) $106 $112 $5 Average salary of ungraded positions $184 $193 $9 Personnel Compensation: 11.1 Full-time permanent $33,440 $36,003 $2,562 11.3 Other than full-time permanent $15,756 $16,615 $859 11.5 Other personnel compensation $2,538 $2,676 $138 11.7 Military personnel $635 $670 $35 11.8 Special personnel services payments $6,278 $6,621 $342 11.9 Subtotal Personnel Compensation $58,648 $62,585 $3,937 12.1 Civilian personnel benefits $22,412 $23,539 $1,127 12.2 Military personnel benefits $78 $83 $4 13.0 Benefits to former personnel 0 0 0 Subtotal Pay Costs $81,138 $86,207 $5,069 21.0 Travel and transportation of persons $992 $1,091 $99 22.0 Transportation of things $107 $109 $3 23.1 Rental payments to GSA 0 0 0 23.2 Rental payments to others 0 0 0 23.3 Communications, utilities and misc. charges $73 $75 $2 24.0 Printing and reproduction $4 $5 0 25.1 Consulting services $80,325 $71,576 -$8,748 25.2 Other services $19,587 $19,218 -$369 25.3 Purchase of goods and services from government accounts $108,960 $109,955 $996 25.4 Operation and maintenance of facilities $515 $516 0 25.5 Research and development contracts $24,347 $23,737 -$610 25.6 Medical care $485 $505 $20 25.7 Operation and maintenance of equipment $6,586 $6,744 $158 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal Other Contractual Services $240,805 $232,251 -$8,553 26.0 Supplies and materials $5,244 $5,370 $126 31.0 Equipment $4,063 $4,161 $98 32.0 Land and structures 0 0 0 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions $1,330,925 $1,334,083 $3,158 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends $13 $13 0 44.0 Refunds 0 0 0 Subtotal Non-Pay Costs $1,582,227 $1,577,158 -$5,069 Total Budget Authority by Object Class $1,663,365 $1,663,365 $0 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses
(Dollars in Thousands) Object Classes FY 2023 Enacted FY 2024 President's Budget FY 2024 +/- FY 2023 Personnel Compensation: Full-time permanent (11.1) $33,440 $36,003 $2,562 Other than full-time permanent (11.3) $15,756 $16,615 $859 Other personnel compensation (11.5) $2,538 $2,676 $138 Military personnel (11.7) $635 $670 $35 Special personnel services payments (11.8) $6,278 $6,621 $342 Subtotal Personnel Compensation (11.9) $58,648 $62,585 $3,937 Civilian personnel benefits (12.1) $22,412 $23,539 $1,127 Military personnel benefits (12.2) $78 $83 $4 Benefits to former personnel (13.0) 0 0 0 Subtotal Pay Costs $81,138 $86,207 $5,069 Travel and transportation of persons (21.0) $992 $1,091 $99 Transportation of things (22.0) $107 $109 $3 Rental payments to others (23.2) 0 0 0 Communications, utilities and miscellaneous charges (23.3) $73 $75 $2 Printing and reproduction (24.0) $4 $5 0 Other Contractual Services: Consultant services (25.1) $26,955 $27,382 $427 Other services (25.2) $19,587 $19,218 -$369 Purchases of goods and services from government accounts (25.3) $66,808 $67,790 $982 Operation and maintenance of facilities (25.4) $515 $516 0 Operation and maintenance of equipment (25.7) $6,586 $6,744 $158 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $120,450 $121,648 $1,198 Supplies and materials (26.0) $5,244 $5,370 $126 Subtotal Non-Pay Costs $126,871 $128,299 $1,427 Total Administrative Costs $208,010 $214,505 $6,496 - Details of Full-Time Equivalent Employment (FTEs)
Office/
DivisionFY 2022
FinalFY 2023 Enacted FY 2024
President's BudgetCivilian Military Total Civilian Military Total Civilian Military Total Office of the Director Direct: 24 0 24 24 0 24 24 0 24 Total: 24 0 24 24 0 24 24 0 24 Division of Extramural Research Direct: 46 0 46 47 1 48 53 1 54 Total: 46 0 46 47 1 48 53 1 54 Office of Management Direct: 28 0 28 26 0 26 28 0 28 Reimbursable: 55 0 55 57 0 57 59 0 59 Total: 83 0 83 83 0 83 87 0 87 Office of Science Policy and Communication Direct: 25 0 25 25 0 25 28 0 28 Total: 25 0 25 25 0 25 28 0 28 Division of Epidemiology, Services and Prevention Research Direct: 30 0 30 29 2 31 31 2 33 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 30 0 30 29 2 31 31 2 33 Division of Neuroscience and Behavior Direct: 26 0 26 26 0 26 27 0 27 Total: 26 0 26 26 0 26 27 0 27 Division of Therapeutics and Medical Consequences Direct: 29 0 29 28 0 28 28 0 28 Total: 29 0 29 28 0 28 28 0 28 Center for the Clinical Trials Network Direct: 13 0 13 13 0 13 13 0 13 Total: 13 0 13 13 0 13 13 0 13 Intramural Research Program Direct: 116 4 120 121 1 122 122 1 123 Reimbursable: 1 0 1 Total: 117 4 121 121 1 122 122 1 123 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 392 4 396 394 4 398 412 4 416 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2020 13.1 2021 13.0 2022 13.0 2023 13.0 2024 13.0 - Detail of Positions
Detail of Positions1 GRADE FY 2022 Final FY 2023 Enacted FY 2024 President's Budget Total, ES Positions 1 1 1 Total, ES Salary $203,700 $213,885 $224,579 GM/GS-15 65 65 65 GM/GS-14 81 81 84 GM/GS-13 99 100 110 GS-12 39 40 44 GS-11 15 15 15 GS-10 0 0 0 GS-9 5 5 5 GS-8 7 7 7 GS-7 2 2 2 GS-6 1 1 1 GS-5 0 0 0 GS-4 0 0 0 GS-3 0 0 0 GS-2 0 0 0 GS-1 0 0 0 Subtotal 315 317 334 Commissioned Corps (42 U.S.C. 207): Assistant Surgeon General 0 0 0 Director Grade 1 0 0 Senior Grade 2 2 2 Full Grade 1 2 2 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 4 4 4 Ungraded 96 96 97 Total permanent positions 319 321 339 Total positions, end of year 416 418 436 Total full-time equivalent (FTE) employment, end of year 396 398 416 Average ES salary $203,700 $213,85 $224,579 Average GM/GS grade 13.0 13.0 13.0 Average GM/GS salary $131,907 $138,502 $145,427 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Budget Graphs
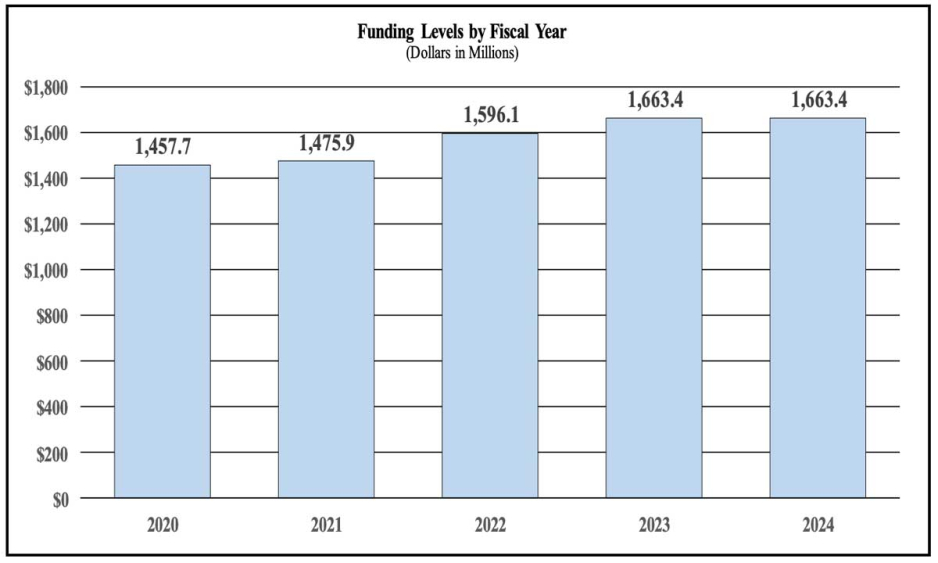
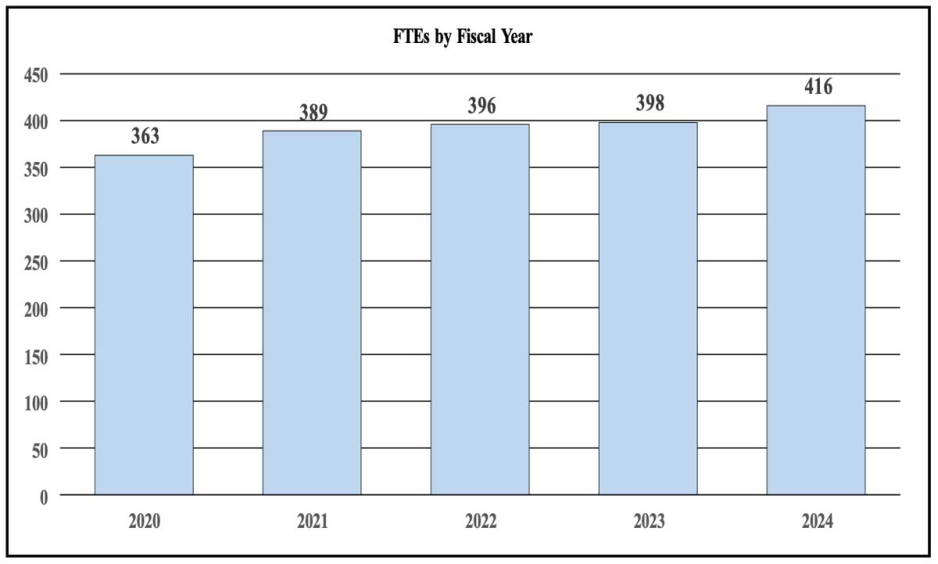
History of Budget Authority and FTEs:
Distribution by Mechanism (dollars in thousands):
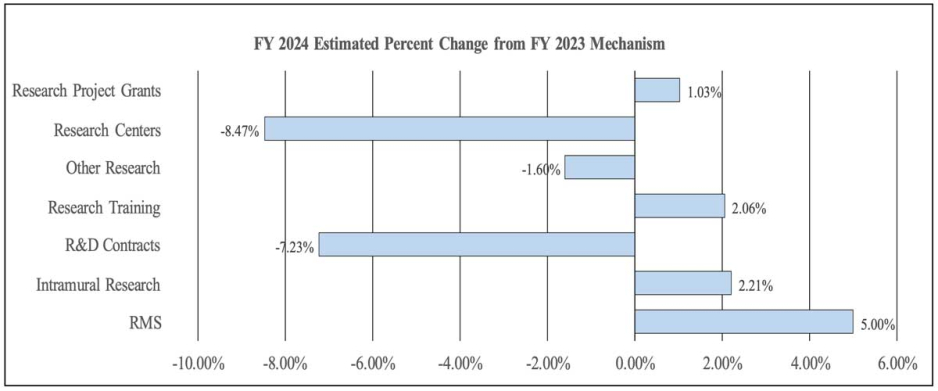
Change by Selected Mechanism:
Justification of Budget Request
National Institute on Drugs and Addiction (NIDA)
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2022 Final | FY 2023 Enacted | FY 2024 President's Budget | FY 2024 +/- FY 2023 | |
|---|---|---|---|---|
| BA | $1,596,123,000 | $1,663,365,000 | $1,663,365,000 | 0 |
| FTE | 396 | 398 | 416 | +18 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2024 President’s Budget request is $1,663.4 million, which is equal to the FY 2023 enacted level. Within this amount, funding for the HEAL Initiative® will remain at $355.3 million, the FY 2023 enacted level.
Major Changes in the Fiscal Year 2024 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below.
Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2024 President’s Budget. The FY 2024 President’s Budget request for NIDA is $1,663.4 million, which is the same as the FY 2023 Enacted level.
- Research Project Grants (RPGs) (+$10.8 million; total $1,059.0 million): The total amount of support to RPG awards will increase by $10.8 million over FY 2023. The number of competing RPG awards will decrease by 38 in comparison to the FY 2023 level. These reductions will be distributed across programmatic areas and basic, epidemiology and/or clinical research.
- Research Centers (-$5.1 million; total $55.0 million): NIDA will continue supporting existing specialized/comprehensive centers projects while selecting three new projects to support.
- Other Research (-$3.2 million; total $193.9 million): NIDA will continue supporting research career development awards, research training awards, and cooperative clinical research at existing levels while making 80 awards for new projects.
- Ruth L Kirchstein Training (+$0.6 million; total $31.0 million): NIDA will increase its numbers of full time training positions to 535, an increase of 2 positions in comparison to the FY 2023 Enacted level.
Directors Overview

Director since 2003
The National Institute on Drugs and Addiction (NIDA)1 supports robust research programs to monitor the shifting landscape of drug use, develop more effective interventions for preventing and treating addiction and overdose, and make these advances available to all Americans. The United States saw more than 100,000 fatal drug overdoses in 2021, an unprecedented loss of life that shows no sign of abating. In the past 5 years, although overdose deaths due to prescription opioids have decreased, there have been increased deaths involving other opioids such as illicit fentanyl, as well as co-use of opioids and stimulants such as methamphetamine.2
While the increased presence of fentanyl in the drug supply is one factor in overdose deaths, persistent stigma against people with substance use disorder (SUD) is likely another, as it can deter them from seeking treatment. Research shows that entrenched ways of communicating about substance use, including the phrase “substance abuse,” can perpetuate this stigma.3 That is why NIDA is proposing to change its name from the “National Institute on Drug Abuse” to the “National Institute on Drugs and Addiction.”
NIDA also recognizes concerning trends in drug use and outcomes among the Nation’s young people. During the COVID-19 pandemic, fatal overdoses among adolescents nearly doubled after a decade of stable overdose rates, mostly due to illicit fentanyl.4 Over the past decade, more permissive state laws have increased access to cannabis for both therapeutic and recreational use; these changes have been associated with increased recreational cannabis use,5 with past-year use by young adults setting records in 2021.6 Nicotine vaping also remained high among young adults in 2021, with about one-fifth reporting past-year use.4
NIDA closely monitors these developments and shifts resources as needed so that the Nation is equipped to prevent and treat substance use disorders (SUD) and overdose. For example, NIDA has begun planning a national registry to monitor cannabis use and to expand our National Drug Early Warning System that tracks emerging substances. NIDA has also enhanced our research on the unique effects of fentanyl and its analogues, and mechanisms of polysubstance addiction. Such efforts are helping us to identify concerning trends in substance use and its outcomes, and to develop earlier, more effective interventions at individual and community levels.
In September 2022, we released a new 7 that will guide our efforts for the next 5 years. The plan reflects our commitment to improve public health by advancing critical areas of addiction research—from fundamental neuroscience and pharmacology, to epidemiology and prevention, to therapeutics development, testing, implementation, and dissemination. The plan also reflects a redoubling of our efforts to translate research into practice; reduce health inequities based on race, ethnicity, and social determinants of health (SDOH); and grow a talented and more diverse workforce in addiction science.
Continued support for fundamental research on drugs and addiction
NIDA supports basic research that increases our understanding of both the rewarding and toxic effects of drug use on the brain and body, including the neurobiological mechanisms of addiction—from the level of genes, cells, and brain circuits to how they interact with social environments throughout the lifespan.In one recent NIDA-funded study on mice, researchers found that there are cell type-specific gene expression patterns in the brain after cocaine exposure. They then used gene editing tools to activate the same patterns in mice never exposed to cocaine, and found that those mice behaved as if they were intoxicated.8 Such gene expression programs could help reveal how and when occasional drug use evolves into SUD, and some of the genes involved could be targets for new prevention and treatment strategies. Other recent projects have led to new tools for mapping the dynamic activity of individual cell signaling molecules in the brain and even within single brain cells.9 Such advances will help us investigate the brain’s responses to drugs and the pathways underlying addiction with unprecedented precision.
NIDA supports two large studies exploring early-life factors and their influence on the developing brain, including the brain circuits involved in SUD. The Adolescent Brain Cognitive Development (ABCD) study is collecting brain imaging and other data from nearly 12,000 children from ages 9-10 to adulthood. It is the largest long-term study of brain development in the United States and has generated hundreds of peer-reviewed reports with implications for the role of early experiences on health over the lifespan. With co-funding from the National Institutes of Health (NIH) Helping to End Addiction Long-term (HEAL)® Initiative and other NIH Institutes and Centers, we also recently launched the HEALthy Brain and Child Development (HBCD) study, which will collect similar data from thousands of children from the prenatal period through their first decade of life. In addition to contributing fundamental knowledge about SUD, these studies are exemplars of multidisciplinary team science. Together, they comprise nearly 50 research sites that bring together experts in neuroscience, psychology, psychiatry, pediatrics, and other disciplines. Moreover, because ABCD data are made available to the broader research community, most ABCD analyses and findings are published by investigators not directly involved in the study.
Developing more effective strategies to prevent and treat SUD and overdose
NIDA is helping drive progress in strategies to prevent and treat opioid and stimulant misuse, especially during youth, when life experiences have a substantial effect on the risk of SUD and interventions are likely to have the most impact. Our prevention portfolio also has a focus on integrating prevention into healthcare delivery systems to reduce the research-to-practice gap and target interventions to settings where they are most needed. In one recent NIDA-funded study, researchers developed an education program to prevent diversion of stimulants prescribed to high school students with attention-deficit/hyperactivity disorder (ADHD). In a pilot study, the program reduced students’ disclosure of their prescription to friends, their intent to share, and being approached to share.10 These researchers continue to explore risk factors for diversion among teens and young adults who take prescription stimulants.
We are also prioritizing investment in novel overdose reversal agents and medications to treat SUD, including longer-lasting formulations of existing medications, novel compounds, immunotherapies, and sequestrants designed to stop drugs from entering the brain. (See Program Portrait “Treating methamphetamine addiction.”) Since September 2019, our medication development portfolio has led to 30 investigational new drugs for OUD submitted to the U.S. Food and Drug Administration (FDA) for authorization to conduct human testing.
We also continue research focused on harm reduction, which aims to reduce the morbidity and mortality associated with substance misuse. Harm reduction approaches, such as syringe services programs (SSP) and distribution of the opioid overdose reversal agent naloxone, have been shown to reduce infection transmission and overdose risk. Yet, because most studies of harm reduction have been limited to urban environments, there is a need to investigate these approaches in rural areas and hard-to-reach populations. To address such issues, with support from the NIH HEAL Initiative®, we recently established a harm reduction research network that will examine novel approaches, settings, and modes of delivery for harm reduction strategies. In addition, we have expanded our support for research focused on the growing need and variety of services designed to help people recover from SUD and avoid relapse. (See Program Portrait “Supporting recovery research.”)
Bringing evidence-based interventions into broader practice
In addition to efforts to develop and improve interventions for substance misuse and overdose, NIDA supports implementation science to ensure that evidence-based interventions reach all people who need them, especially historically underserved communities. For example, the HEALing Communities Study is testing an integrated model of evidence-based care that includes increasing access to medications for opioid use disorder (OUD) and overdose in nearly 70 communities hit hard by the opioid crisis. The Justice Community Opioid Innovation Network, which is also funded through the NIH HEAL Initiative®, is testing approaches to improve care for people with OUD in carceral settings. Through HEAL we have expanded our implementation science portfolio to address other gaps in the continuum of care for people with OUD. For example, a new program announced in 2022 focuses on improving SUD care for patients served by community health centers (CHCs), including testing new strategies for CHC-based screening and referral to drug treatment programs. Another new program, HEAL Data2Action, aims to improve the use of real-time data to identify gaps in healthcare services for OUD and overdose, and to deploy evidence-based interventions to fill those gaps. NIDA also continues to support research on the use of telehealth to provide SUD treatment to hard-to-reach populations—work that was amplified during the COVID-19 pandemic. (See Program Portrait “Exploring telehealth for SUD care.”)
Addressing health disparities and social determinants of health (SDOH)
Compared to White people, people of color with SUD are less likely to receive treatment, and those arrested for drug charges are more likely to be incarcerated. The COVID-19 pandemic brought new disparities—including a surge in fatal overdoses among Black and American Indian/Alaska Native (AI/AN) people, in part related to SDOH including access to treatment.11
In 2020, NIDA established its Racial Equity Initiative (REI) to address the impact of structural racism on addiction science and to promote racial equity within NIDA’s research community and programs. After a series of listening sessions, surveys, and meetings to engage the NIDA community in setting goals for the initiative, we published an REI Action Plan, which aims in part to enhance research on racial inequities in substance use, addiction, and related outcomes.
In FY 2022, the REI released a suite of nine funding opportunities to further this goal. Focus areas include inequities in substance use and HIV outcomes in underserved racial/ethnic groups; and understanding how structural racism affects cognition, mental health, and risk of SUD. NIDA-funded research is also helping address health inequities by investigating how SDOH across the lifespan affect the odds of SUD and treatment outcomes. For example, one project is examining the impact of SDOH on SUD risk and recovery across three generations in eight AI/AN communities. Another project is leveraging the ABCD study to determine if early-life SDOH lead to changes in brain structure and function that predict substance misuse. In addition, a new program funded through the HEAL Initiative® will focus on developing interventions that target SDOH to prevent opioid misuse, OUD, and comorbidities.
Building and sustaining a talented workforce that reflects the Nation’s diversity
We recognize that racism and unconscious bias have an insidious effect on the research community, to the detriment of NIDA’s research mission. The REI Action Plan therefore includes goals to improve diversity and inclusion at NIDA and in the extramural workforce, and we are putting these goals into action. For example, REI FY 2022 funding opportunities include support for capacity building at minority-serving institutions, which are uniquely positioned to engage minority and underserved communities and translate research into community-tailored practices. As part of the REI, we also announced targeted support for new and at-risk investigators, particularly those who are underrepresented in addiction science.
NIDA’s established diversity programs also continue to grow and foster a more diverse workforce that reflects our Nation’s diversity. For example, our Research Education Program grants provide funds for established researchers to recruit, train, and mentor early-career researchers from underrepresented groups in addiction science. The program currently funds 14 sites across 10 states, including 3 sites added in FY 2022. For 25 years, the NIDA Summer Research Internship Program has focused on enhancing undergraduate diversity in science, technology, engineering, and math (STEM) by placing diverse students in labs across the United States. In a survey of the 2021 class, over 70 percent reported they were more likely to pursue a PhD in STEM as a result of their experience.12
References
- The FY 2024 President’s Budget proposes to rename the National Institute on Drug Abuse to the National Institute on Drugs and Addiction.
- pubmed.ncbi.nlm.nih.gov/33965972
- pubmed.ncbi.nlm.nih.gov/20005692
- pubmed.ncbi.nlm.nih.gov/35412573
- pubmed.ncbi.nlm.nih.gov/35618659; pubmed.ncbi.nlm.nih.gov/36002928
- monitoringthefuture.org/wp-content/uploads/2022/08/mtfpanelchap2_2022.pdf
- nida.nih.gov/about-nida/noras-blog/2022/09/nida-releases-its-2022-2026-strategic-plan
- pubmed.ncbi.nlm.nih.gov/32637607
- pubmed.ncbi.nlm.nih.gov/32926777; pubmed.ncbi.nlm.nih.gov/34764251
- pubmed.ncbi.nlm.nih.gov/31185307
- pubmed.ncbi.nlm.nih.gov/36125815; www.cdc.gov/mmwr/volumes/71/wr/mm7129e2.htm
- pubmed.ncbi.nlm.nih.gov/35902690
Program Descriptions
Division of Neuroscience and Behavior (DNB)
The Division of Neuroscience and Behavior (DNB) supports research to understand the biological mechanisms that underlie drug use and addiction, and to inform the development of novel prevention and treatment strategies for SUD. This includes identifying the effects of illicit drugs on brain structure and function throughout the lifespan; and how genes, the environment, and other factors influence the risk of SUD and its outcomes. DNB also supports research on drug pharmacology; non-pharmacological SUD treatments; data science; and technology that enables study of the living brain from cells to circuits to networks.
With support from the NIH HEAL Initiative®, NIDA and other NIH Institutes and Centers, DNB administers the HBCD study, which will examine the neurologic, cognitive, social, and emotional development of about 7,500 children from the prenatal period to age 10. Before the study began recruiting families in late 2021, it had to address the challenges of conducting magnetic resonance imaging (MRI) with young children. One challenge was how to keep infants asleep and still during MRI, for which HBCD investigators developed an MRI-compatible crib that can rock infants to sleep and then position them in the MRI scanner without disturbing them. Investigators also surveyed families living near HBCD study sites and found differences in potential barriers and incentives to their participation. For example, free childcare and playgroups during study visits were more incentivizing to Black respondents than white respondents. These data are helping investigators implement recruitment strategies that will ensure diverse participation in the study.1
DNB supported several recent studies that have found molecular and cellular targets for potential SUD therapies. One study explored the possibility that medications used to treat high blood pressure—called angiotensin-converting enzyme (ACE) inhibitors—might hold clues to treating addiction. The investigators found that in mice, ACE inhibitors stimulate certain natural opioids (endorphins) and counteract the addictive effects of fentanyl, suggesting the potential to redesign and repurpose them for treating SUD.2 Other NIDA-funded research has produced evidence that cells called astrocytes play a protective role in addiction. Astrocytes surround neurons and can “vacuum up” the chemical signals that neurons release, providing a kind of circuit breaker. Recent studies show that astrocytes respond dynamically to opioid exposure by moving closer to synapses and turning up their vacuum power.3 Therapeutics that boost these responses could help treat or prevent SUD.
DNB also supported an innovative new approach to screen massive virtual chemical libraries. Such libraries are a trove of potential therapeutics but screening them can be time- and cost-prohibitive. The new approach, called V-SYNTHES, starts by screening virtual chemical fragments for their ability to engage a target (e.g., a receptor). Fragments that show the strongest engagement are pursued by adding modular pieces to them and screening them again in repeated cycles. The inventors of V-SYNTHES used it to screen a virtual library of some 11 billion compounds and identified 21 compounds that bind to brain cannabinoid receptors.4
Budget Policy: The FY 2024 President’s Budget request is $534.0 million, a decrease of $2.4 million or 0.5 percent compared with the FY 2023 enacted level.
Supporting recovery research
A variety of community-based services have evolved to help people navigate recovery from SUD by making healthy, goal-directed changes to their lives. These recovery services include peer-based mutual aid groups, recovery housing, and youth programs, and many provide linkages to social services and vocational training. They tend to have strong foundations in the lived experience of people with SUD, yet there is a lack of knowledge about which services are most effective for different people.
NIDA recognizes that the recovery research field needs foundational support to advance the evidence base for effective, personalized recovery services. In 2020, NIDA began funding recovery research networks to develop and disseminate outcome measures, tools, resources, and training programs to support growth in the recovery research field. Through the HEAL Initiative®, NIDA expanded this program in 2022 to fund additional research networks and help coordinate their activities through a Consortium on Addiction Recovery Science (CoARS).
CoARS researchers will develop resources for research and training related to a variety of recovery models, including youth programs, recovery housing, and recovery community centers that combine professional SUD treatment with peer-led services. The centers are working with diverse populations, including Black and Latino communities, justice-involved individuals, and rural Americans. The expanded program also funds projects that will develop recovery interventions in collaboration with people with lived experience and design clinical trials to test these approaches, including peer interventions to help individuals start and maintain MOUD. Through these efforts, NIDA is helping drive research to generate evidence-based recovery services for people from all backgrounds.
Division of Epidemiology, Services, and Prevention Research (DESPR)
The Division of Epidemiology, Services, and Prevention Research (DESPR) supports research to understand and address the interactions between individuals and environments that contribute to drug use, addiction, and related health problems. DESPR supports a broad portfolio that informs evidence-based strategies to support prevention, harm reduction, treatment, and recovery for people at risk or with SUDs. This includes two nationally representative studies—the Monitoring the Future (MTF) survey, which measures substance use and related attitudes among adolescents, and the Population Assessment of Tobacco and Health (PATH) Study, which focuses on tobacco use, attitudes, and health outcomes of people aged 12 and older.
MTF and PATH continue to add to our understanding of trends in substance use and their impact on health. For example, while past studies suggested that most teens reduce drug use as they enter adulthood, MTF recently found that teens with symptoms of severe SUD were likely to experience such symptoms in adulthood. The PATH Study recently analyzed use of e-cigarettes (e-cigs) and health outcomes among adults, and found that for smokers of conventional cigarettes who have no intention to stop, e-cigs may help them reduce their smoking or quit over time. However, consistent with other research, PATH has also found that compared to smokers and never-smokers, e-cig users have an intermediate risk of short-term respiratory problems such as wheezing and cough.5 The long-term health risks of e-cig use remain unknown.
DESPR also supports research examining the efficacy and implementation of harm reduction efforts, including reducing the risk of HIV and hepatitis C virus (HCV) infection associated with injection drug use. SSPs provide sterile syringes, HIV and HCV testing, and linkage to treatment for these conditions and for SUD, but have a limited capacity to reach rural areas. To address this gap, NIDA-funded researchers developed a system wherein SSPs use telehealth to connect patients to an HIV specialist. In a pilot study, 35 people received antiretroviral therapy through this intervention, and of those, nearly 80 percent had clinically suppressed HIV levels at 6 months.6 A large trial of this intervention is now underway.
DESPR also supports the ABCD study, which is following children from ages 9-10 to adulthood to identify risk factors for SUD. Recently, the study explored differences in brain structure associated with alcohol use disorder (AUD), which were long theorized to be caused by alcohol toxicity. But the investigators found that among children never exposed to alcohol, those with genetic risk factors for AUD were likely to have the brain differences previously only seen in adults with AUD. Thus, rather than being a consequence of AUD, those differences could predispose people to AUD and could help inform preventive strategies.7 Another analysis from the ABCD study found that children whose mothers had used cannabis after the first 5-6 weeks of pregnancy were more likely to have social, behavioral, and attentional problems at age 11-12.8 This adds to the evidence that cannabis use during pregnancy can adversely affect prenatal development, with impacts for the child’s health many years into the future. In addition to its focus on early-life substance exposures and SUD risk factors, the ABCD study has led to broader advances in understanding child health, including the impact of the COVID-19 pandemic on children’s mental health and the importance of sleep in brain development.9
Budget Policy: The FY 2024 President’s Budget request is $372.6 million, a decrease of $1.7 million or 0.5 percent compared with the FY 2023 enacted level.
Treating methamphetamine addiction
The prevalence of methamphetamine use disorder (MUD) and overdose deaths involving methamphetamine have risen sharply in recent years.1 Although there is currently no FDA-approved medication for MUD, NIDA is investing in research on a variety of potential treatments for MUD and strategies to bring effective treatments to the communities that need them most.
NIDA supports research exploring pharmacological treatments for MUD. For example, the CTN found that a combination of injectable naltrexone and oral bupropion, a commonly prescribed medication for depression and nicotine cessation, successfully reduced meth use and cravings in people with MUD.2 Other CTN trials are testing buprenorphine, ketamine, or transcranial magnetic stimulation to treat MUD and co-occurring polysubstance use. NIDA-funded researchers are also developing innovative new therapies, such as small-molecule sequestrants and a monoclonal antibody to trap methamphetamine in blood and reduce its entry into the brain. This treatment has received fast track designation from the FDA and is being tested for safety in people with MUD.
NIDA-funded research has helped establish the efficacy of contingency management (CM), which is currently the most effective evidence-based treatment available for stimulant use disorders including MUD. CM is a behavioral therapy that involves patients receiving tangible rewards, such as gift cards for groceries, to reinforce healthful behaviors such as abstinence. Current studies continue to explore the efficacy of CM and other behavioral interventions for MUD, as well as more impactful ways of bringing these interventions to people with MUD, such as peer-to-peer treatment or meth sobering centers. NIDA-funded trials are also evaluating whether smartphone-based CM can reduce stimulant use and HIV risk among gay and bisexual men. Continued investments in these research areas will help fill the gap in evidence-based treatments for MUD.
References
- pubmed.ncbi.nlm.nih.gov/34550301
- pubmed.ncbi.nlm.nih.gov/33497547
Division of Therapeutics and Medical Consequences (DTMC)
The Division of Therapeutics and Medical Consequences (DTMC) supports research to evaluate the safety and efficacy of pharmacotherapies, behavioral interventions, and medical devices to prevent and treat SUDs and drug overdose. This work spans all phases of medical product development including synthesis and preclinical evaluation of potential therapeutics, clinical trial design and execution, and preparing regulatory submissions.
DTMC supports the development of new medications for SUD, as well as the repurposing of drugs currently used to treat other conditions. For example, among people recovering from OUD, sleep disturbances are often part of withdrawal and can increase the risk of relapse. But taking common sleep aids like sedatives could further increase the risk of relapse and overdose. Thus, DTMC supports research on unique sleep medications that target orexins, proteins in the brain that help promote wakefulness and also modulate dopamine-producing brain cells, which drive the rewarding effects of drugs. Preliminary results show that an orexin receptor blocker, suvorexant, reduces withdrawal and improves sleep for people with OUD.10
Among current studies on behavioral interventions is a project to improve treatment for chronic pain and depression associated with OUD. These conditions affect 40-60 percent of people with OUD and can increase the risk of opioid misuse if not treated.11 Researchers are developing an approach in which primary care providers and behavioral health specialists will collaborate to treat such patients.
Funding from the NIH HEAL Initiative® has enabled NIDA to expand its medication development portfolio, including support for research on new types of medications for opioid use disorder (MOUD). Currently available MOUD such as methadone, buprenorphine, and naltrexone can reduce opioid misuse and lower the risk of overdose, but these medications generally must be taken on a rigorous schedule to avoid relapse. To address this, NIDA-funded clinical trials are testing subcutaneous implants of extended-release naltrexone that are designed to last for months. Oral extended-release levomethadone is also being evaluated as a safer, more accessible alternative to methadone. DTMC also supports development of novel biologics to treat SUD, such as monoclonal antibodies designed to neutralize drugs before they reach the brain. Additionally, DTMC supports research on neuromodulation therapies to correct the activity of brain circuits involved in addiction. For example, a current trial is evaluating the feasibility of treating OUD with deep brain stimulation, which is FDA-approved for Parkinson’s disease and severe epilepsy.
Budget Policy: The FY 2024 President’s Budget request is $129.3 million, a decrease of $0.6 million or 0.5 percent compared with the FY 2023 enacted level.
Center for Clinical Trials Network (CTN)
The Center for Clinical Trials Network (CTN) provides a collaborative framework for healthcare providers, researchers, and patients to conduct clinical trials on the safety and efficacy of SUD interventions. The CTN includes 16 research nodes across the country and more than 240 community-anchored treatment programs. This unique structure enables the CTN to investigate behavioral, pharmacological, and integrated therapies across diverse settings and populations, and to develop implementation strategies that help bring research results into practice. Active protocols focus on a variety of areas, including primary prevention of SUD; increasing patient access and adherence to MOUD, especially in rural and underserved populations; evaluating potential medications for stimulant use disorder; and addressing stigma and other barriers to SUD treatment. Some examples are highlighted below.
Among the 2.5 million people who had OUD in 2020, only 11.2 percent received MOUD.12 Because people with OUD often receive acute care for overdose or other conditions in the emergency department, this presents an opportunity for starting MOUD treatment. A CTN study found that providing high-dose buprenorphine during emergency care was safe for patients with OUD who did not respond well to low doses—an approach that may help such patients control cravings and withdrawal and engage in follow-up care.13
The CTN is exploring many other approaches to expand patients’ access to MOUD. For example, because most people visit their community pharmacist more often than they see their doctor, the CTN tested a physician-pharmacist collaborative model of care. In that study, about 70 adults with OUD were transitioned from physician management of buprenorphine to management by their pharmacy. Among the 90 percent of patients who completed the study, 95 percent adhered to buprenorphine treatment.14 The CTN is studying the potential for community pharmacies to provide other MOUD types and to conduct OUD screening and referrals.
The CTN also recently explored the association between OUD and depression, and how patients with both conditions respond to MOUD. In a study of nearly 600 patients with OUD, nearly half had depression when they started MOUD. After four weeks, two-thirds of those patients improved in their depression, but those with severe depression were less likely to improve.15 The findings suggest that patients with OUD should be screened for depression and that when depression does not improve after MOUD, additional therapies may be needed.
Budget Policy: The FY 2024 President’s Budget request is $40.8 million, a decrease of $0.2 million or 0.5 percent compared with the FY 2023 enacted level.
Exploring Telehealth for SUD Care
The COVID-19 pandemic brought increased rates of drug overdose coupled with limitations on in-person healthcare—creating an urgent need to provide SUD treatment remotely. To that end, federal emergency authorities were used to expand SUD telehealth care, helping save lives during the pandemic.
Importantly, NIDA-funded research shows that telehealth also holds promise for the future of SUD treatment. For example, observational studies found that telehealth programs help engage and retain people in MOUD treatment.1 And a large study of Medicare beneficiaries confirmed that, compared to pre-pandemic rates, expansion of telehealth during the pandemic was associated with better treatment retention and lower overdose risk.2 Studies on patient experiences with telehealth have found that it reduces barriers such as the need for transportation and the stigma associated with visiting SUD treatment facilities.3 Meanwhile, a survey of MOUD providers found that 85 percent were in favor of permanently extending telehealth flexibilities.4 Such findings support the benefits and sustainability of SUD telehealth care far beyond the pandemic.
In ongoing research, NIDA is addressing challenges to scaling up SUD telehealth care. For example, NIDA-funded studies have found that low digital literacy and internet access, low referral rates to telehealth, and provider capacity barriers remain significant barriers.5 Moreover, these barriers disproportionately impact underserved populations, including rural-dwelling, low-income, and disabled individuals. The HEALing Communities Study and other NIDA-supported efforts are developing strategies to expand equitable access to SUD telehealth care, for example by improving provider telehealth knowledge and resources.
References
- pubmed.ncbi.nlm.nih.gov/34890927
- pubmed.ncbi.nlm.nih.gov/36044198
- pubmed.ncbi.nlm.nih.gov/35443862
- pubmed.ncbi.nlm.nih.gov/36121359
- pubmed.ncbi.nlm.nih.gov/33879260; 34517225; 35895282
Office of Translational Initiatives and Program Innovations (OTIPI)
The Office of Translational Initiatives and Program Innovations (OTIPI) translates discoveries in addiction research into candidate health applications. OTIPI supports translational research through NIDA’s Small Business Innovation Research/Technology Transfer (SBIR/STTR) programs, as well as Challenge competitions. OTIPI also develops training programs that help scientists move their discoveries from the lab to the real world.
OTIPI supports innovative addiction research and therapeutics development by startup companies. For example, recognizing the therapeutic potential of psychedelic drugs such as psilocybin, in FY 2023, NIDA announced a new program to support small businesses to develop psychedelic-based therapies for SUD. In the telehealth field, NIDA has funded online systems that connect people to addiction treatment and related services. This includes apps to deliver interventions such as cognitive behavioral therapy, enable people to manage MOUD through virtual care, and maintain 24/7 engagement with recovery and relapse prevention services.
Through OTIPI, NIDA also funds new technologies to measure community substance use patterns through wastewater monitoring. This includes funding for Biobot, which uses an algorithm to select sewer access sites (manholes) that will best represent community substance use, then deploys a robotic device inside each manhole to collect samples, and tests for opioids and other drugs in those samples using standard laboratory methods. During the COVID-19 pandemic, Biobot technology was also used to test community levels of SARS-CoV-2 in wastewater, including in a collaboration with the NIH Rapid Acceleration of Diagnostics–Underserved Populations (RADx-UP) program.16 NIDA also funds development of lab-on-a-chip technology that was recently shown to detect opioids in wastewater with similar sensitivity to standard methods. This platform holds potential for rapid, cost-effective measurement, without the need to take samples to a lab for processing.17
OTIPI also supports development of technology to reduce prescription drug diversion, including by healthcare workers. While rates of such diversion are unknown, most hospitals attempt to reduce diversion by using automated drug dispensing cabinets with monthly audits to detect anomalous dispensing. Unfortunately, those systems are slow and prone to error and manipulation. To develop a more effective system, NIDA-funded scientists developed artificial intelligence-powered software that monitors automated dispensing cabinets and employee time clocks to detect potential diversion in real time. The researchers have tested their system against a historical dataset of about 28 million drug transactions including 22 known diversions; it detected all of them, at an average 160 days faster than the time taken for actual discovery.18
Finally, OTIPI has coordinated several recent Challenge competitions to take on complex problems in addiction science by seeking innovative solutions from the public, in addition to the scientific community. For example, the “Start an SUD Startup” Challenge invited competitors to propose a startup venture focused on a novel product or approach to address drug addiction. Winning proposals, announced in January 2022, included apps to connect recovery support specialists to clients and peers; portable devices and wearables to detect fentanyl and other substances; and neuromodulation therapy combining music and tactile stimulation. Each winning team received $10,000 and entrepreneurial mentorship, with the goal that some will form startups that can compete successfully for SBIR or STTR funding. Other recent Challenges focused on development of product prototypes to combat drug craving and novel postmortem toxicology tools to improve investigation of suspected drug overdose deaths.
Budget Policy: The FY 2024 President’s Budget request is $42.7 million, a decrease of $0.2 million or 0.5 percent compared with the FY 2023 enacted level.
The NIH HEAL Initiative®
The NIH HEAL Initiative® was launched in 2018 to accelerate scientific solutions to the national opioid overdose crisis, including improved treatment strategies for pain as well as OUD. NIDA coordinates several innovative HEAL programs that are developing and testing evidence-based interventions for opioid misuse and overdose in diverse populations and settings.
The HEALing Communities Study is testing an integrated model of evidence-based care to reduce overdose deaths in 67 communities hit hard by the opioid crisis. The study has three core components: (1) a menu of evidence-based practices (EBPs) designed to increase the use of MOUD, widen distribution of naloxone, and reduce high-risk opioid prescribing; (2) community engagement to select the EBPs and strategies that best meet each community’s needs; and (3) communications to address stigma about OUD and disseminate EBPs.19
The Justice Community Opioid Innovation Network (JCOIN) is studying approaches to improve evidence-based treatment for people with OUD in justice settings, including prisons and jails. It is estimated that half of incarcerated individuals have an SUD and that only about one in four receive any SUD treatment.20 Recent JCOIN studies show that ensuring access to MOUD in prisons and jails could significantly reduce overdose deaths and recidivism among incarcerated people in the years following their release.21 Ongoing JCOIN studies are evaluating strategies to help recently incarcerated people find and engage in SUD treatment in their communities.22
A new harm reduction research network will develop, test, and implement strategies to prevent overdose, transmission of HIV and HCV, and other harms associated with drug use. The network includes a coordinating center and nine projects focused on a variety of strategies and outcomes, including delivery of harm reduction services during emergency care, via mobile vans for hard-to-reach patients, and in combination with peer-based contingency management.
Another new program, HEAL Data2Action (HD2A), is supporting research to help health systems build real-time data analytics capacity to identify and address service gaps in prevention and treatment of OUD, recovery support, and harm reduction. HD2A currently funds projects focused on areas such as clinical decision support for chronic pain, improving overdose fatality review, stabilizing people at risk for overdose through linkage to MOUD treatment, and improving MOUD access and treatment retention through coordinated care and safe take-home methadone dosing. HD2A also assists researchers with data infrastructure, implementation of evidence-based solutions to service gaps, and long-term sustainability of these solutions.
Budget Policy: The FY 2024 President’s Budget request is $355.3 million, which is equal to the FY 2023 enacted level. This includes $11.2 million for Research Management and Support related to this area of research.
Intramural Research Program (IRP)
The NIDA Intramural Research Program (IRP) conducts state-of-the-art basic, preclinical, and clinical research to inform strategies for prevention and treatment of SUD and related health outcomes. The IRP portfolio includes research to elucidate the mechanisms underlying development of SUDs, evaluate potential new therapies, and identify and characterize emerging drugs such as synthetic opioids, stimulants, and cannabinoids.
IRP scientists led a recent study to better understand brain mechanisms of reward and reinforcement, which are disrupted in addiction.23 The scientists used MRI to examine brain activity in mice given rewards for pressing a lever, and in people as they watched TikTok videos associated with binge-watching. By initially focusing on a brain region called the prefrontal cortex (PFC), which has previously been implicated in addiction, they found that the PFC is part of a positive feedback circuit that is activated by reinforcing stimuli. The findings could help guide future use of neuromodulation therapies to adjust the brain circuitry underlying addiction.
The Neuroimaging Core contributed to another study with implications for neuromodulation therapies. This study investigated why accidental brain lesions (e.g., from a stroke) sometimes cause smokers to quit.24 Previously, such lesions have been found in many brain regions, and none were consistently associated with smoking cessation. By mapping the lesions for their connectivity to other brain areas, the study identified a brain circuit that, when damaged, is associated with reduced addiction to nicotine and alcohol. This circuit, which includes parts of the PFC, could be an ideal target for neuromodulation.
Other IRP scientists are conducting innovative research on dopamine signaling to develop potential new medications for SUD. While drugs that block the function of dopamine D3 receptors offer the potential to treat SUD, they also have adverse effects on the heart. The scientists, who helped solve the structure of the D3 receptor 10 years ago, have used that structure to design new more selective compounds. They have found that two such compounds reduce craving for opioids and cocaine in rodent models of addiction without cardiotoxicity.25
Budget Policy: The FY 2024 President’s Budget request is $113.8 million, an increase of $2.5 million or 2.2 percent compared with the FY 2023 enacted level.
Research Management and Support (RMS)
Research Management and Support (RMS) activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Staff supported by NIDA’s RMS budget also coordinate training and career development programs to sustain a talented, diverse workforce of addiction scientists. Other RMS functions include strategic planning, coordination, dissemination of latest research findings and funding opportunities, program evaluation, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. RMS staff also play key roles in coordinating NIDA’s involvement in the NIH HEAL Initiative® and in managing HEAL-supported research. In addition to the infrastructure required to support research and training, NIDA strives to provide evidence-based resources and educational materials about substance use and addiction. To this end, the RMS portfolio includes education and outreach activities to inform health policy and practice, and to provide the public with timely, accessible, trustworthy information about drug research in English and Spanish. In addition, the RMS portfolio includes the NIDAMED initiative, which is aimed at engaging and educating clinicians in the latest addiction science.
Budget Policy: The FY 2024 President’s Budget request is $86.1 million, an increase of $4.1 million or 5.0 percent compared with the FY 2023 enacted level.

References
- pubmed.ncbi.nlm.nih.gov/34242880
- pubmed.ncbi.nlm.nih.gov/35201898
- pubmed.ncbi.nlm.nih.gov/34888837; pubmed.ncbi.nlm.nih.gov/35947652
- pubmed.ncbi.nlm.nih.gov/34912117
- pubmed.ncbi.nlm.nih.gov/34962556; pubmed.ncbi.nlm.nih.gov/34304335
- pubmed.ncbi.nlm.nih.gov/34781096
- pubmed.ncbi.nlm.nih.gov/34092032
- pubmed.ncbi.nlm.nih.gov/36094599
- pubmed.ncbi.nlm.nih.gov/35090817; pubmed.ncbi.nlm.nih.gov/35914537
- pubmed.ncbi.nlm.nih.gov/35731889
- pubmed.ncbi.nlm.nih.gov/12746360; pubmed.ncbi.nlm.nih.gov/28476267
- www.samhsa.gov/data/report/2020-nsduh-annual-national-report
- pubmed.ncbi.nlm.nih.gov/34264326
- pubmed.ncbi.nlm.nih.gov/33428284
- pubmed.ncbi.nlm.nih.gov/35452194
- pubmed.ncbi.nlm.nih.gov/34863144
- pubmed.ncbi.nlm.nih.gov/34863144
- pubmed.ncbi.nlm.nih.gov/35136913
- pubmed.ncbi.nlm.nih.gov/33248391
- nap.nationalacademies.org/read/25310/chapter/6; pubmed.ncbi.nlm.nih.gov/34304335
- pubmed.ncbi.nlm.nih.gov/32712165; pubmed.ncbi.nlm.nih.gov/35063323
- pubmed.ncbi.nlm.nih.gov/33531212
- pubmed.ncbi.nlm.nih.gov/35296648
- pubmed.ncbi.nlm.nih.gov/35697842
- pubmed.ncbi.nlm.nih.gov/27508895; pubmed.ncbi.nlm.nih.gov/30555159; pubmed.ncbi.nlm.nih.gov/31562201