*The FY 2025 President’s Budget proposes to rename the National Institute on Drug Abuse to the National Institute on Drugs and Addiction.
Directors Overview

Director since 2003
The National Institute on Drugs and Addiction (NIDA) supports research to understand the shifting landscape of drug use; develop more effective interventions for preventing and treating addiction, overdose, and other drug-related harms; and make these advances available to all Americans. According to provisional data through March 2023, over 110,000 Americans are dying from drug overdoses each year, driven in large part by synthetic opioids (such as fentanyl) and stimulants (such as methamphetamine and cocaine), with increasing levels of combined (polysubstance) use. Often unknown to the consumer, fentanyl is increasingly adulterated with novel substances such as xylazine—a veterinary tranquilizer that has been designated as an emerging drug threat by the White House. While these are sobering developments, NIDA has moved swiftly to counteract them and save lives; in the last year alone, NIDA-funded research helped improve the availability of new and existing overdose reversal agents, with many more therapeutics for overdose and substance use disorder (SUD) in the pipeline. Research also shows that entrenched ways of communicating about substance use, including the phrase “substance abuse,” can perpetuate stigma, which is why NIDA is proposing to change its name from the “National Institute on Drug Abuse” to the “National Institute on Drugs and Addiction.”
Through the NIH Helping to End Addiction Long-term (HEAL) Initiative® in FY 2023, NIDA has launched a number of new programs to prevent and treat SUD and overdose. These include research to improve prevention and treatment services for polysubstance use, and to develop new interventions for polysubstance use disorders. (See “Multi-pronged strategies to address multiple substance use.”) As part of the White House xylazine response plan, NIDA is funding new research to understand, prevent, and treat xylazine-related harms, including severe flesh wounds that can lead to amputation. NIDA also is expanding its research on approaches such as wastewater-based epidemiology to gather real-time, comprehensive data on community levels of xylazine and other emerging substances. (See “Pioneering research on wastewater testing to detect drugs and infectious diseases.”)
NIDA also tracks changing attitudes about substance use and their impact on public health. For example, e-cigarettes, cannabis, and certain psychedelic drugs are increasingly perceived as low risk despite their addiction liability and potential long-term harms, especially among youth. NIDA continues to fund the Monitoring the Future (MTF) survey, which measures substance use and related attitudes among adolescents, and the Population Assessment of Tobacco and Health (PATH) Study, which focuses on tobacco use, attitudes, and health outcomes of people aged 12 and older. In addition, NIDA recently funded a cannabis registry that will capture data on cannabis product use and health outcomes, and conduct testing on products associated with adverse outcomes. NIDA is also funding research to better understand the effects of psychedelic drugs, their potential for treating SUD, and the impact of shifting policies in this area.
Driving discovery through fundamental research
NIDA continues to support fundamental, investigator-initiated research into how licit and illicit drugs affect the brain and body. These investments lay a foundation for understanding mechanisms of SUD, its prevention and treatment, and approaches to harm reduction and recovery. For example, basic research on opioids is yielding insights into their effects on pain and their potential to lead to SUD and overdose—which may ultimately lead to more effective, less risky opioid analgesics. In a structure-function study of opioids and the mu opioid receptor (the primary binding site for fentanyl and other opioids), researchers found that compared to morphine, fentanyl occupies unique sub-pockets of the receptor, activating unique cell signaling pathways. This may help explain why fentanyl and its analogs are 100-10,000 times more potent than morphine.1 In another study, these researchers used structure-function analyses to design synthetic opioid analgesics with lower risks of addiction and overdose.2 Continued basic research also lays a foundation that enables addiction science to shift rapidly to new challenges when necessary. For example, as xylazine spread across the United States in 2022, NIDA scientists who study the body’s responses to opioids were able to pivot their work to study how adding xylazine to opioids affects brain function and opioid-induced respiratory suppression.
Untangling complex SUD risk factors to develop more effective prevention
In alignment with the Federal Overdose Prevention Strategy, NIDA has increased its focus on primary prevention of substance use and SUD, especially during early life. Childhood and adolescence are critical periods for learning and brain development when life experiences can become etched into malleable brain circuits. In fact, it is known that early-life exposures to trauma and substance use are independently associated with SUD and other behavioral health disorders in adulthood. Largely unknown, however, is why such exposures translate to negative long-term outcomes for some young people and not others—and how best to intervene for at-risk youth.
With support from other NIH Institutes and Centers, NIDA leads two large studies exploring early-life factors and their influence on brain health throughout the lifespan. Since 2016, the Adolescent Brain Cognitive Development (ABCD) study has been collecting brain imaging, genetic, and other data from nearly 12,000 young people, starting at ages 9-10. Funded in part by the HEAL Initiative, the HEALthy Brain and Child Development (HBCD) study will collect similar data from thousands of children starting in the prenatal period through adolescence. These studies promise unprecedented insight into brain development and vulnerability to mental illness, including SUD. In fact, one recent analysis using ABCD data suggests that diverse mental health disorders share a common feature—a failure to prune excess brain connections in early life. This hyper-connectivity could one day serve as a biomarker to support early interventions for mental illness.3 Other recent findings show that social determinants of health (SDOH), such as family income, can have powerful effects on brain development and mental health and that policy interventions can mitigate these effects.
NIDA also supports research to translate knowledge about SUD risk factors into primary prevention. For example, a series of projects focuses on targeting SDOH to prevent opioid use disorder (OUD) and co-occurring mental health conditions. This includes a study on blight remediation efforts—such as cleaning up vacant lots, abandoned buildings, and dilapidated homes—and whether this can reduce rates of opioid misuse and overdose in Philadelphia. Other projects are evaluating SUD prevention interventions for high-risk youth, including those living with psychiatric disorders, transitioning out of carceral settings, or experiencing homelessness. In partnership with other NIH components and Federal health agencies, NIDA is also supporting a consensus study by the National Academies of Sciences, Engineering, and Medicine to develop actionable recommendations to address the prevention research-to-practice gap and establish the infrastructure needed to implement SUD and other behavioral health prevention interventions.
Advancing SUD treatment, harm reduction, and recovery
The increasing diversity and potency of illicit drugs requires diverse, powerful treatments for SUD and overdose. NIDA has answered this need by greatly expanding its SUD therapeutics development portfolio; since September 2019, NIDA-supported research has led to more than 50 Investigational New Drug applications and 2 Investigational Device Exemptions submitted to the Food and Drug Administration (FDA) for evaluation in clinical trials.
NIDA-funded research led to the first naloxone nasal spray, approved by the FDA in 2015 as an effective medication to reverse opioid overdose. Years of follow-up research established that wider distribution of naloxone, especially to people who use drugs, could reduce community overdose rates, helping lead to FDA approval of the first non-prescription versions of naloxone in 2023. More recent NIDA investments helped lead to a nasal spray formulation of nalmefene, another opioid-blocking agent, which also received FDA approval in 2023. Nalmefene lasts hours longer than naloxone—which is not always sufficient, even after more than one dose, to fully reverse overdoses that involve increasingly powerful opioids.
NIDA is increasing support for therapies using psychedelic drugs, such as psilocybin (from “magic mushrooms”) and dissociative drugs, such as ketamine. While these substances carry risk, there is evidence that when used in clinical settings in combination with psychotherapy, they can help people recover from SUD. In FY 2023, NIDA announced it would commit up to $1.5 million to advance research on psychedelic-based therapies. This work is part of NIDA’s broader therapeutics development portfolio, which encompasses diverse strategies such as immunotherapies, sequestrants, repurposed medications, neuromodulation therapies, and others. (See “Accelerating therapeutics development for drug addiction and overdose.”)
Harm reduction and recovery are pillars of the Federal Overdose Prevention Strategy and significant focus areas of NIDA-funded research. Harm reduction approaches include, but are not limited to, naloxone distribution, syringe services programs (SSP), and tools such as fentanyl test strips (FTS) for checking whether drugs contain fentanyl. Among ongoing harm reduction projects, the HEALing Communities Study is testing strategies to increase FTS and naloxone uptake in diverse, high-risk communities, and to measure the impact on overdose rates. Another study aims to better understand people’s experiences with xylazine to inform harm reduction strategies.
With HEAL Initiative funding in FY 2022, NIDA launched a new research network focused on harm reduction. Network priorities include testing new harm reduction strategies, evaluating new ways to deliver existing strategies, and reaching underserved populations. In addition to data on opioids, stimulants, and other commonly misused drugs, the network is also collecting data on xylazine exposures. HEAL funding also enabled NIDA to expand an existing recovery research network that aims to build evidence for effective addiction recovery support services.
Expanding access to SUD interventions and addressing racial-ethnic disparities
NIDA supports implementation research on how to effectively deploy proven prevention and treatment approaches in diverse healthcare settings and beyond—including community, education, social service, and justice settings—in order to reach people at highest risk of SUD and poor outcomes. For example, the HEALing Communities Study aims to reduce rates of OUD and overdose in nearly 70 communities through an integrated model of evidence-based care that includes increasing access to medications for OUD (MOUD), such as methadone, buprenorphine (BUP), and naltrexone. Researchers have worked closely with participating communities to select and deploy those practices most likely to have an impact in each one.4 The Justice Community Opioid Innovation Network (JCOIN) supports research and capacity building to strengthen connections between the justice system and SUD treatment systems. JCOIN has found that ensuring access to MOUD in prisons and jails could reduce overdose deaths among recently incarcerated people by one-third.5 NIDA plans to extend JCOIN in FY 2025 to test large-scale implementation of OUD interventions across justice settings.
In recent years, risk of overdose mortality has increased steeply among Black Americans and American Indian/Alaska Natives (AI/AN), although absolute overdose numbers remain highest among whites. In FY 2022, as part of its Racial Equity Initiative (REI), NIDA released a suite of funding opportunities to enhance research on racial inequities in substance use, addiction, and related outcomes. One funded project is testing a culturally informed, peer-led intervention to connect Black individuals to BUP treatment at community health centers. In partnership with the Shawnee Tribe, another project is using neuroimaging to examine how the brain responds to tribal traditions and culture that are associated with substance use prevention. With HEAL funding and in close partnership with other NIH Institutes, Centers, and Offices, NIDA and the National Institute of Neurological Disorders and Stroke (NINDS) are supporting a new program focused on AI/AN and Native Hawaiian health called the Native Collective Research Effort to Enhance Wellness (N-CREW). N-CREW researchers will work in partnership with AI/AN Tribes and Native American-serving organizations to identify community-based priorities related to overdose, substance use, mental health, and pain in Native communities; collaborate in research toward culturally grounded interventions; and build local capacity as needed for this research.
Supporting a talented diverse cadre of addiction scientists
Since ending the overdose epidemic will require multi-faceted solutions, NIDA funds a variety of training and career development programs to empower emerging scientists from diverse backgrounds to bring their talents to bear on substance use and addiction. NIDA continues to prioritize early-career support, including special consideration for research applications from Early-Stage Investigators. In addition, NIDA participates in a HEAL-funded NIH Pathway to Independence Award program in pain and SUD research intended to help postdoctoral scientists transition to independence. NIDA also has increased its NIH Loan Repayment Program funding to relieve student debt for early-career scientists of limited means.
NIDA sees a critical need for an addiction science workforce that reflects the Nation’s diversity. At the same time, this workforce should represent diverse and growing disciplines, such as data science. Thus, in FY 2023, NIDA announced new programs designed to help diverse students and postdoctoral researchers cross-train in addiction science, Big Data, and artificial intelligence (AI). One program will support research education activities, and another will support student research projects at institutions that do not receive substantial funding from NIH. Program applicants are required to submit plans to increase trainee diversity. Other NIDA programs helping to foster a diverse addiction workforce include the Diversity Scholars Network and the NIDA Summer Research Internship Program.
References
- https://pubmed.ncbi.nlm.nih.gov/36411392
- https://pubmed.ncbi.nlm.nih.gov/36450356
- https://pubmed.ncbi.nlm.nih.gov/37095248
- https://pubmed.ncbi.nlm.nih.gov/36123106
- https://pubmed.ncbi.nlm.nih.gov/32712165
IC Fact Sheet
Major Changes in the Fiscal Year 2025 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below.
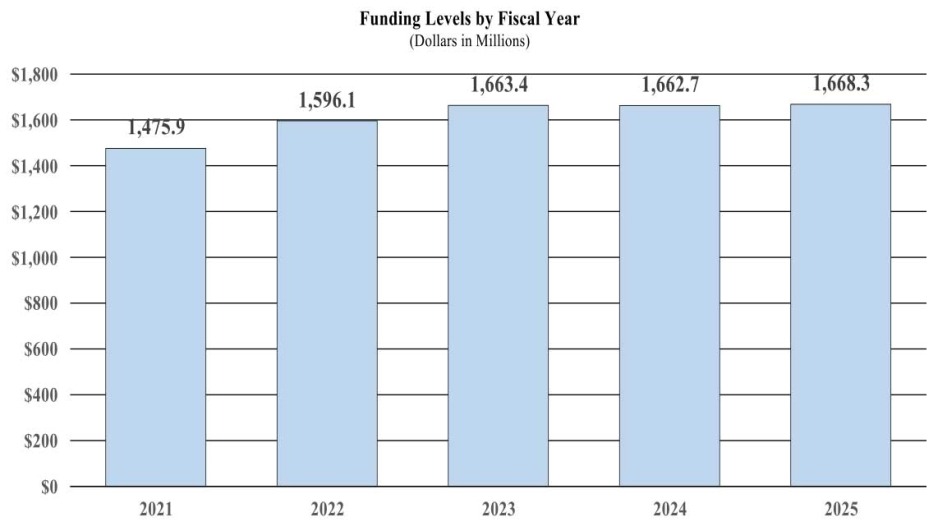
Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2025 President’s Budget. The President’s Budget request for the National Institute on Drugs and Addiction (NIDA) is $1,668.3 million, which is a $5.0 million increase over FY 2023 Final level.
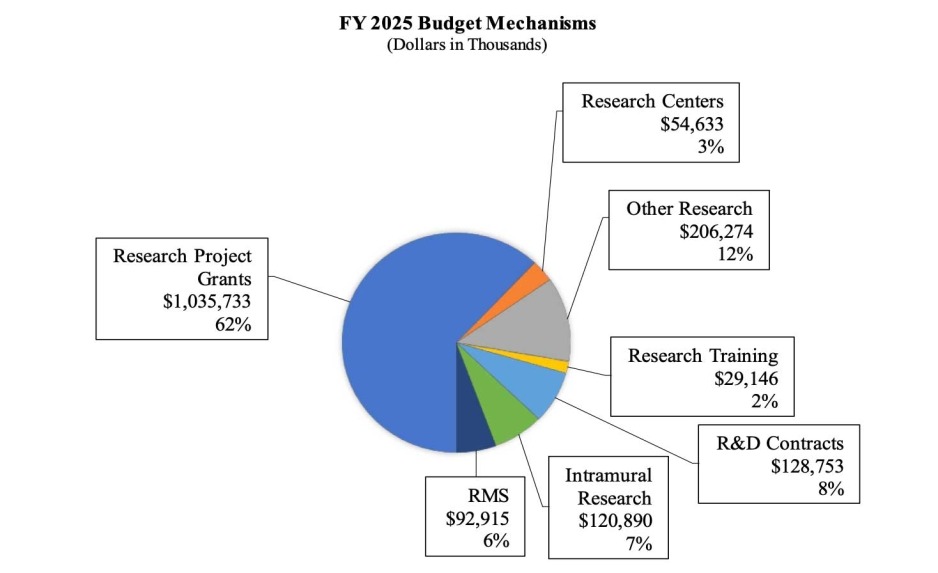
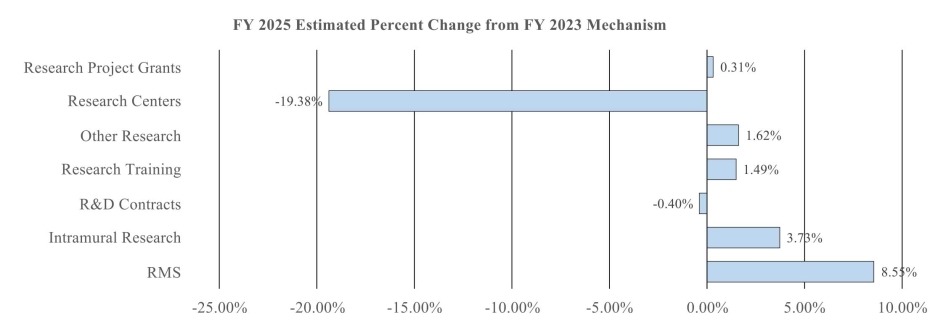
- Research Project Grants (RPGs) (+$3.2 million; total $1,035.7 million): The total amount of support to RPG awards will increase by $3.2 million over the FY 2023 Final level. The number of competing RPG awards will decrease by 93 in comparison to FY 2023. These reductions in competing and noncompeting awards will be distributed across programmatic areas and basic, epidemiology and/or clinical research.
- Research Centers (-$13.1 million; total $54.6 million): NIDA will continue supporting existing specialized/comprehensive centers projects. Potential savings in this area will be applied to support other mechanisms.
- Other Research (+$3.3 million; total $206.3 million): NIDA will continue supporting research career development awards, research training awards, and cooperative clinical research projects.
- Ruth L Kirchstein Training (+$0.4 million; total $29.1 million): NIDA will increase its numbers of full-time training positions to 539, an increase of 7 positions in comparison to the FY 2023 Final level.
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to drugs and addiction, $1,668,343,000.
Tables
- Budget Mechanism
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2023
FinalFY 2024 CR FY 2025
President's
BudgetFY 2025
+/-
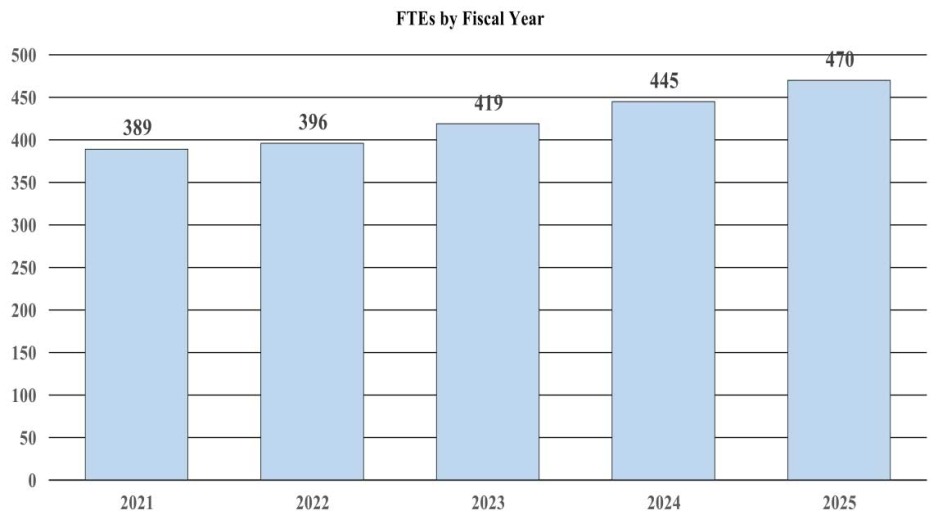
FY 2023No. Amount No. Amount No. Amount No. Amount Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Research Projects: Noncompeting 952 $670,130 990 $731,846 909 $732,968 -43 $62,838 Administrative Supplements (107) $16,305 (92) $14,000 (92) $14,000 (-15) -$2,305 Competing: Renewal 27 $14,861 27 $15,000 29 16,000 2 $1,139 New 371 $279,071 264 $215,337 277 $221,721 -94 -$57,350 Supplements 1 $108 0 0 0 0 -1 -$108 Subtotal, Competing 399 $294,039 291 $230,337 306 $237,721 -93 -$56,318 Subtotal, RPGs 1,351 $980,473 1,281 $976,183 1,215 $984,688 -136 $4,125 SBIR/STTR 78 $52,012 79 $52,581 77 $51,045 -1 -$967 Research Project Grants 1,429 $1,032,485 1,360 $1,028,764 1,292 $1,035,733 -137 $3,248 Research Centers: Specialized / Comprehensive 30 $67,767 26 $58,539 24 $54,633 -6 -$13,134 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 0 0 0 0 0 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 30 $67,767 26 $58,539 24 $54,633 -6 -$13,134 Other Research: Research Careers 262 $49,623 266 $50,346 268 $50,688 6 $1,065 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 28 $83,766 28 $83,606 28 $83,171 0 -$596 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 $2,039 0 $1,984 0 $1,371 0 -$668 Other 84 $67,565 87 $70,249 87 $71,044 3 $3,480 Other Research 374 $202,993 381 $206,185 383 $206,274 9 $3,281 Total Research Grants 1,833 $1,276,382 1,829 $1,305,337 1,843 $1,307,870 14 $2,533 Ruth L Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Individual Awards 178 $8,172 179 $8,360 182 $8,544 4 $373 Institutional Awards 354 $20,545 354 $20,766 357 $20,601 3 $56 Total Research Training 532 $28,717 533 $29,126 539 $29,146 7 $429 Research and Development Contracts 109 $129,265 109 $132,506 110 $128,753 1 -$512 (SBIR/STTR) (non-add) (1) ($1,753) (0) ($685) (1) ($2,187) (0) ($434) Intramural Research 125 $116,541 125 $118,519 125 $120,890 0 $4,349 Res. Management & Support 294 $85,597 320 $89,055 345 $92,915 51 $7,318 Res. Management & Support (SBIR Admin) (non-add) ($92) ($209) ($220) ($128) Construction 0 0 0 0 0 0 0 0 Buildings and Facilities 0 0 0 0 0 0 0 0 Total, NIDA 419 $1,663,365 445 $1,662,695 470 $1,668,343 51 $4,978 1 All numbers in italics and brackets are non-add entries.
- Amounts Available for Obligation
(Dollars in Thousands)1 Source of Funding FY 2023
FinalFY 2024
CRFY 2025
President's
BudgetAppropriation $1,662,695 $1,662,695 $1,668,343 Mandatory Appropriation (non-add) Type 1 Diabetes ($0) ($0) ($0) Other Mandatory financing ($0) ($0) ($0) Subtotal, adjusted appropriation $1,662,695 $1,662,695 $1,668,343 OAR HIV/AIDS Transfers $670 $0 $0 Subtotal, adjusted budget authority $1,663,365 $1,662,695 $1,668,343 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $1,663,365 $1,662,695 $1,668,343 Unobligated balance lapsing -$46 0 0 Total obligations $1,663,319 $1,662,695 $1,668,343 1Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2023 - $15,468, FY 2024 - $15,000 FY 2025 - $15,000
- Summary of Changes
(Dollars in Thousands) CHANGES FY 2023 Final FY 2025 President's Budget Built-In Change from
FY 2023 FinalFTEs/Budget Authority FTEs/Budget Authority FTEs/Budget Authority 1. Intramural Research: A. Built-in cost changes: a. FY 2024 effect of FY 2023 pay & benefits increase $33,010 $35,840 $389 b. FY 2024 effect of FY 2024 pay & benefits increase $33,010 $35,840 $1,285 c. FY 2024 paid days adjustment $33,010 $35,840 $127 d. Differences attributable to FY 2024 change in FTE $33,010 $35,840 $0 e. FY 2025 effect of FY 2024 pay & benefits increase $33,010 $35,840 $436 f. FY 2025 effect of FY 2025 pay & benefits increase $33,010 $35,840 $587 g. FY 2025 paid days adjustment $33,010 $35,840 $0 h. Differences attributable to FY 2025 change in FTE $33,010 $35,840 $0 i. Payment for centrally furnished services $12,601 $13,512 $910 j. Cost of laboratory supplies, materials, other expenses, and non-recurring costs $70,927 $71,538 $4,306 Subtotal, IR built-in cost changes $8,040 2. Research Management and Support: A. Built-in cost changes: a. FY 2024 effect of FY 2023 pay & benefits increase $49,199 $54,926 $582 b. FY 2024 effect of FY 2024 pay & benefits increase $49,199 $54,926 $1,914 c. FY 2024 paid days adjustment $49,199 $54,926 $189 d. Differences attributable to FY 2024 change in FTE $49,199 $54,926 $5,375 e. FY 2025 effect of FY 2024 pay & benefits increase $49,199 $54,926 $647 f. FY 2025 effect of FY 2025 pay & benefits increase $49,199 $54,926 $888 g. FY 2025 paid days adjustment $49,199 $54,926 $0 h. Differences attributable to FY 2025 change in FTE $49,199 $54,926 $4,914 i. Payment for centrally furnished services $5,159 $5,531 $373 j. Cost of laboratory supplies, materials, other expenses, and non-recurring costs $31,196 $32,458 $1,823 Subtotal, RMS built-in cost changes $16,704 Summary of Changes - Continued
(Dollars in Thousands)CHANGES FY 2023 Final FY 2025 President's Budget Program Change from FY 2023 Final No. Amount No. Amount No. Amount B. Program: 1. Research Project Grants: a. Noncompeting 952 $686,434 909 $746,968 -43 $60,533 b. Competing 399 $294,039 306 $237,721 -93 -$56,318 c. SBIR/STTR 78 $52,012 77 $51,045 -1 -$967 Subtotal, RPGs 1,429 $1,032,485 1,292 $1,035,733 -137 $3,248 2. Research Centers 30 $67,767 24 $54,633 -6 -$13,134 3. Other Research 374 $202,993 383 $206,274 9 $3,281 4. Research Training 532 $28,717 539 $29,146 7 $429 5. Research and development contracts 109 $129,265 110 $128,753 1 -$512 Subtotal, Extramural $1,461,227 $1,454,538 -$6,689 FTEs FTEs FTEs 6. Intramural Research 125 $116,541 125 $120,890 0 -$3,691 7. Research Management and Support 294 $85,597 345 $92,915 51 -$9,386 8. Construction 0 0 0 9. Buildings and Facilities 0 0 0 Subtotal, program changes -$19,766 Total built-in and program changes 419 $1,663,365 470 $1,668,343 51 $4,978 - Authorizing Legislation
PHS Act/Other Citation U.S.Code Citation 2024 Amount Author-
izedFY 2024 CR 2025 Amount Author
-izedFY 2025 President's Budget Research and Investigation Section 301 42§241 Indefinite $1,662,695,000 Indefinite $1,668,343,000 National Institute on Drugs and Addiction Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,662,695,000 $1,668,343,000 - Appropriations History
Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2016 $1,047,397,000 $1,050,875,000 $1,069,086,000 $1,077,488,000 Rescission $0 20171 $1,050,550,000 $1,107,700,000 $1,103,032,000 1,090,853,000 Rescission $0 2018 $864,998,000 $1,107,497,000 $1,113,442,000 $1,383,603,000 Rescission $0 2019 $1,137,403,000 $1,400,126,000 $1,420,591,000 $1,419,844,000 Rescission $0 2020 $1,296,379,000 $1,489,237,000 $1,490,498,000 $1,462,016,000 Rescission $0 2021 $1,431,770,000 $1,476,590,00 $1,505,192,000 $1,479,660,000 Rescission $0 2022 $1,852,503,000 $1,860,329,000 $1,832,906,000 $1,595,474,000 Rescission $0 2023 $1,843,326,000 $1,712,832,000 $1,684,230,000 $1,662,695,000 Rescission $0 2024 $1,663,365,000 $1,662,695,000 $1,672,695,000 $1,662,695,000 Rescission $0 2025 $1,668,343,000 1Budget Estimate to Congress includes mandatory financing.
- Budget Authority by Activity
(Dollars in thousands)1 Extramural Research FY 2023 Final FY 2024 CR FY 2025
President's
BudgetFY 2025
+/-
FY 2023 FinalFTE Amount FTE Amount FTE Amount FTE Amount Detail: Division of Therapeutics and Medical Consequences $110,301 $109,851 $109,842 -$459 Division of Neuroscience and Behavior $534,398 $532,218 $532,174 -$2,224 Division of Epidemiology, Services and Prevention Research $392,721 $391,119 $391,087 -$1,634 Center for the Clinical Trials Network $35,842 $35,695 $35,693 -$149 Office of Translational Initiatives and Program Innovations $43,419 $43,242 $43,238 -$181 HEAL Initiative2 $344,547 $342,995 $342,505 -$2,042 Subtotal, Extramural $1,461,227 $1,455,120 $1,454,538 -$6,689 Intramural Research 125 $116,541 125 $118,519 125 $120,890 0 $4,349 Research Management & Support 294 $85,597 320 $89,055 345 $92,915 51 $7,318 TOTAL 419 $1,663,365 445 $1,662,695 470 $1,668,343 51 $4,978 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Total for HEAL Initiative including RMS is (in thousands) $345,295 in FY 2022, $355,295 in FY 2023, and $355,295 in FY 2024.- Budget Authority by Object Class
(Dollars in Thousands)1 OBJECT CLASSES FY 2024 CR FY 2025 President's Budget Total compensable workyears: Full-time equivalent 445 470 Full-time equivalent of overtime and holiday hours 1 1 Average ES salary $191 $195 Average GM/GS grade 13.0 13.0 Average GM/GS salary $144 $148 Average salary, Commissioned Corps (42 U.S.C. 207) $163 $170 Average salary of ungraded positions $144 $148 Personnel Compensation: 11.1 Full-time permanent $39,741 $42,351 11.3 Other than full-time permanent $15,018 $15,438 11.5 Other personnel compensation $2,114 $2,173 11.7 Military personnel $547 $573 11.8 Special personnel services payments $7,633 $7,847 11.9 Subtotal Personnel Compensation $65,053 $68,382 12.1 Civilian personnel benefits $21,618 $22,341 12.2 Military personnel benefits $40 $42 13.0 Benefits to former personnel 0 0 Subtotal Pay Costs $86,711 $90,766 21.0 Travel and transportation of persons $1,221 $1,248 22.0 Transportation of things $48 $49 23.1 Rental payments to GSA 0 0 23.2 Rental payments to others $3 $3 23.3 Communications, utilities and misc. charges $72 $73 24.0 Printing and reproduction $0 $0 25.1 Consulting services $85,946 $84,538 25.2 Other services $26,260 $25,238 25.3 Purchase of goods and services from government accounts $110,812 $111,236 25.4 Operation and maintenance of facilities $1,812 $1,812 25.5 Research and development contracts $7,980 $8,155 25.6 Medical care $564 $586 25.7 Operation and maintenance of equipment $3,907 $3,993 25.8 Subsistence and support of persons 0 0 25.0 Subtotal Other Contractual Services $237,281 $235,559 26.0 Supplies and materials $6,108 $6,135 31.0 Equipment $8,551 $8,640 32.0 Land and structures 0 0 33.0 Investments and loans 0 0 41.0 Grants, subsidies and contributions $1,322,700 $1,325,870 42.0 Insurance claims and indemnities 0 0 43.0 Interest and dividends 0 0 44.0 Refunds 0 0 Subtotal Non-Pay Costs $1,575,984 $1,577,577 Total Budget Authority by Object Class $1,662,695 $1,668,343 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses
(Dollars in Thousands) Object Classes FY 2024 CR FY 2025 President's Budget Personnel Compensation: Full-time permanent (11.1) $39,741 $42,351 Other than full-time permanent (11.3) $15,018 $15,438 Other personnel compensation (11.5) $2,114 $2,173 Military personnel (11.7) $547 $573 Special personnel services payments (11.8) $7,633 $7,847 Subtotal Personnel Compensation (11.9) $65,053 $68,382 Civilian personnel benefits (12.1) $21,618 $22,341 Military personnel benefits (12.2) $40 $42 Benefits to former personnel (13.0) 0 0 Subtotal Pay Costs $86,711 $90,766 Travel and transportation of persons (21.0) $1,221 $1,248 Transportation of things (22.0) $48 $49 Rental payments to others (23.2) $3 $3 Communications, utilities and miscellaneous charges (23.3) $72 $73 Printing and reproduction (24.0) $0 $0 Other Contractual Services: Consultant services (25.1) $70,401 $72,152 Other services (25.2) $26,260 $25,238 Purchases of goods and services from government accounts (25.3) $99,591 $100,015 Operation and maintenance of facilities (25.4) $1,812 $1,812 Operation and maintenance of equipment (25.7) $3,907 $3,993 Subsistence and support of persons (25.8) 0 0 Subtotal Other Contractual Services $201,971 $203,211 Supplies and materials (26.0) $6,108 $6,135 Subtotal Non-Pay Costs $209,423 $210,719 Total Administrative Costs $296,134 $301,485 - Details of Full-Time Equivalent Employment (FTEs)
Office/
DivisionFY 2023 Final FY 2024 CR FY 2025 President's Budget Civilian Military Total Civilian Military Total Civilian Military Total Office of the Director Direct: 25 0 25 27 0 27 29 0 29 Total: 25 0 25 27 0 27 29 0 29 Division of Extramural Research Direct: 55 1 56 60 1 61 62 1 63 Total: 55 1 56 60 1 61 62 1 63 Office of Management Direct: 82 0 82 86 - 86 92 0 92 Reimbursable: - - - - - - - - - Total: 82 0 82 86 0 86 92 0 92 Office of Science Policy and Communication Direct: 29 0 29 29 0 29 31 0 31 Total: 29 0 29 29 0 29 31 0 31 Division of Epidemiology, Services and Prevention Research Direct: 32 2 34 36 2 38 38 2 40 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 32 2 34 36 2 38 38 2 40 Division of Neuroscience and Behavior Direct: 28 0 28 29 0 29 31 0 31 Total: 28 0 28 29 0 29 31 0 31 Division of Therapeutics and Medical Consequences Direct: 27 0 27 28 0 28 30 0 30 Total: 27 0 27 28 0 28 30 0 30 Center for the Clinical Trials Network Direct: 13 0 13 14 0 14 14 0 14 Total: 13 0 13 14 0 14 14 0 14 Intramural Research Program Direct: 123 1 124 131 1 132 138 1 139 Reimbursable: 1 - 1 1 - 1 1 - 1 Total 124 1 125 132 1 133 139 1 140 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 415 4 419 441 4 445 466 4 470 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2021 13.0 2022 13.0 2023 13.2 2024 13.2 2025 13.2 - Detail of Positions
Detail of Positions1 GRADE FY 2023 Final FY 2024 CR FY 2025 President's Budget Total, ES Positions 1 1 1 Total, ES Salary $181,544 $190,984 $194,804 GM/GS-15 63 68 72 GM/GS-14 90 97 103 GM/GS-13 115 124 133 GS-12 42 46 48 GS-11 14 15 16 GS-10 0 0 0 GS-9 5 5 6 GS-8 6 6 7 GS-7 2 2 2 GS-6 1 1 1 GS-5 4 4 5 GS-4 1 1 1 GS-3 0 0 0 GS-2 0 0 0 GS-1 0 0 0 Subtotal 343 369 394 Commissioned Corps (42 U.S.C. 207): Assistant Surgeon General 0 0 0 Director Grade 0 0 0 Senior Grade 3 3 3 Full Grade 1 1 1 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 4 4 4 Ungraded 92 92 92 Total permanent positions 347 373 398 Total positions, end of year 440 466 491 Total full-time equivalent (FTE) employment, end of year 419 445 470 Average ES salary $181,544 $190,984 $194,804 Average GM/GS grade 13.2 13.2 13.2 Average GM/GS salary $137,104 $144,233 $147,118 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism (dollars in thousands):
Change by Selected Mechanism:
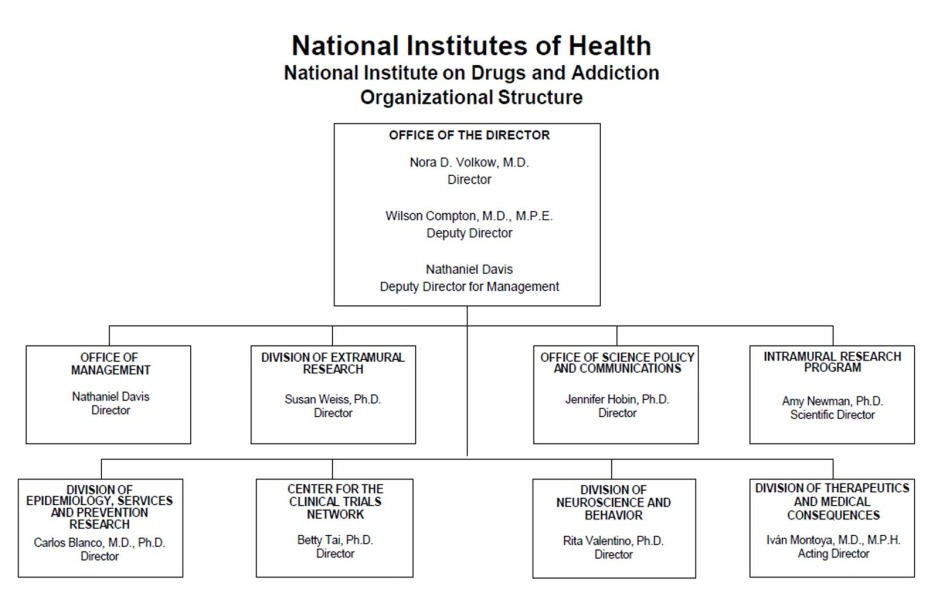
Organizational Chart
- Text Description of Organizational Chart
Office of the Director
- Nora D. Volkow, M.D.
Director - Wilson Compton, M.D., M.P.E.
Deputy Director - Nathaniel Davis
Deputy Director for Management
Office of Management
- Nathaniel Davis
Director
Division of Extramural Research
- Susan Weiss, Ph.D.
Director
Office of Science Policy and Communications
- Jennifer Hobin, Ph.D.
Director
Intramural Research Program
- Amy Newman, Ph.D.
Scientific Director
Division of Epidemiology, Services and Prevention Research
- Carlos Blanco, M.D., Ph.D.
Director
Center for the Clinical Trials Network
- Betty Tai, Ph.D.
Director
Division of Neuroscience and Behavior
- Rita Valentino, Ph.D.
Director
Division of Therapeutics and Medical Consequences
- Ivan Montoya, M.D., M.P.H.
Acting Director
- Nora D. Volkow, M.D.
Justification of Budget Request
National Institute on Drugs and Addiction (NIDA)
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2023 Final | FY 2024 CR | FY 2025 President's Budget | FY 2025 +/- FY 2023 | |
|---|---|---|---|---|
| BA | $1,663,365,000 | $1,662,695,000 | $1,668,343,000 | +$4,978,000 |
| FTE | 419 | 445 | 470 | +51 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2025 President’s Budget request for the National Institute on Drugs and Addiction (NIDA) is $1,668.3 million, which is $5.0 million above the FY 2023 Final level. Within this amount, funding for the Helping to End Addiction Long-term (HEAL) Initiative® will remain at $355.3 million, the FY 2023 Final level. NIDA will continue to support basic and applied research on substance use and addiction—including building the addiction science workforce—toward improving prevention, treatment, recovery, and harm reduction related to substance use, so that all Americans can live healthier lives.
Program Descriptions
Division of Neuroscience and Behavior (DNB)
The Division of Neuroscience and Behavior (DNB) supports research to understand the biological mechanisms that underlie drug use and addiction, and to inform the development of novel prevention and treatment strategies for substance use disorders (SUD). This includes identifying the effects of illicit drugs on brain structure and function throughout the lifespan, and how genes, the environment, and other factors influence the risk of SUD and its outcomes. DNB also supports research on the pharmacology of drugs with addiction potential; data science; foundations of neuromodulation technology; and research tools to enable the study of the living brain from cells to circuits to networks.
A recent study exemplifies DNB support for research on the genetics of addiction. In that study, researchers combed through genomic data from over 1 million people, including children participating in the Adolescent Brain Cognitive Development (ABCD) study, and found nearly 20 gene variants associated with SUD, regardless of the substance involved. The researchers then developed a genetic risk score that was able to discern between healthy individuals and people with an SUD diagnosis, with the highest accuracy for those who had polysubstance use disorder.1 Although genetic approaches like this cannot fully predict who will develop SUD, they could someday aid in SUD risk assessment, lead to more personalized interventions, and inform potential targets for new SUD therapeutics.
Multi-pronged strategies to address multiple substance use
Polysubstance use is a growing factor in fatal overdose and other adverse outcomes. In 2021, about one in three overdose deaths involved both fentanyl and stimulants. Moreover, people with opioid addiction and a co-occurring substance use disorder (SUD) have lower odds of staying in treatment with medications for opioid use disorder (MOUD).
NIDA leads several research efforts to better understand polysubstance use and to develop more effective multi-pronged interventions for it. A program initiated in FY 2023 supports research to understand the neurobiology of polysubstance use and its effects on drug craving, addiction, and other outcomes. For example, one project is examining how interactions between fentanyl and xylazine affect the risk of overdose and responses to treatment.
One program funded with HEAL Initiative dollars supports research to define polysubstance use patterns and outcomes, and to improve prevention and treatment. One project is examining how changes in housing status and other factors affect patterns of combined fentanyl and stimulant use among people experiencing homelessness. Clinical trials funded through the program will investigate interventions for co-use of opioids and stimulants, including contingency management (CM) delivered by a smartphone app. CM involves providing small financial incentives for abstinence and other recovery-oriented behaviors and is currently the only intervention effective for reducing stimulant use. Two trials will examine the potential for integrating smartphone-based CM into MOUD treatment programs, reducing substance use, and improving MOUD adherence.
Another study also supported with HEAL funds is the Co-Care Study, in which NIDA’s Clinical Trials Network is investigating whether team-based collaborative care in primary healthcare settings—a common approach used to manage chronic conditions—can help reduce polysubstance use and overdose risk. Finally, a new program co-led by NIDA and other Institutes using HEAL funds will support research to inform safe and effective medication-based, psychosocial, and complementary interventions for people with co-occurring opioid use disorder and alcohol use disorder.
Since people who use drugs are at higher risk for HIV infection, DNB also supports fundamental research on HIV. With funding through a data science program, one recent study investigated how HIV manages to persist in some people despite antiretroviral therapy, and found that HIV-infected cells sometimes multiply and continue producing the virus, acting as a viral reservoir.2 With funding through the NIDA Avante-Garde program, another study identified genes that are down-regulated during HIV-related brain inflammation, suggesting a potential early step in HIV-associated neurocognitive disorder, which affects up to half of people with HIV.3 Both of these programs were renewed in FY 2023.
DNB also supports research focused on the intersection of SUD and sleep disorders, which have a strong bidirectional association. Substance use can lead to sleep disturbances, and sleep disturbances can increase the risk of drug withdrawal, craving, and relapse. In fact, a recent DNB-funded study found that SUD and sleep disturbances are linked at a molecular level; compared to healthy individuals, people with opioid use disorder (OUD) had unique daily rhythmic patterns of gene activity in the brain, specifically in brain regions involved in addiction.4 In FY 2023, DNB announced new funding to explore the mechanisms that link sleep, circadian rhythms, and SUDs.
Budget Policy: The FY 2025 President’s Budget request is $532.2 million, a decrease of $2.2 million compared with the FY 2023 Final level.
The Division of Epidemiology, Services, and Prevention Research
The Division of Epidemiology, Services, and Prevention Research (DESPR) supports studies to understand and address the interactions between individuals and environments that contribute to drug use, addiction, and related health problems. DESPR also supports epidemiologic research that informs the development of evidence-based prevention, harm reduction, treatment, and recovery interventions, including the Monitoring the Future (MTF) survey and the Population Assessment of Tobacco and Health (PATH) Study.
DESPR’s research is shedding light on e-cigarette use among youth and its relation to smoking and other substance use. For example, many studies suggest that e-cigarette use, or vaping, in adolescence is a gateway to smoking later. New PATH data suggest that vaping also entrenches smoking among teens who have already tried it.5 Meanwhile, MTF has found that compared to teens who do not use nicotine, odds of cannabis use are higher among those who vape nicotine—about 40 times higher among those who both vape and smoke nicotine.6 Even accounting for smoking and cannabis use, youth who use e-cigarettes are more likely to experience wheezing, bronchitis, and shortness of breath.7 These findings highlight the need for interventions to address the health risks of vaping during youth.
DESPR also supports the ABCD study, which is advancing knowledge about the impacts of social determinants of health (SDOH) on brain development. In one analysis, researchers found that Black children ages 9-10 faced greater adversity than white children—including lower parental education and income, living in disadvantaged neighborhoods, and more exposure to trauma such as vehicle accidents and assault—and that this adversity was associated with differences in brain structure. When the differences in adversity were controlled statistically, the brain differences largely evaporated.8 Another analysis found that compared to children from high-income families, those from low-income families were likely to have structural brain differences as well as symptoms of anxiety and depression. More importantly, those disparities narrowed significantly among children living in states with robust antipoverty programs.9 These studies provide real-world evidence that intervening on SDOH can help ensure healthy brain development.
DESPR also supports implementation science to identify and address gaps in translating evidence-based interventions into practice. For instance, a study of national Medicare data found that adults who received buprenorphine (BUP) following an overdose had a 62 percent lower risk of fatal overdose over the next year. However, fewer than 1 in 20 patients received it, and most recipients waited more than 30 days.10 DESPR is funding research on innovative solutions to this challenge, such as collaborative care models that bring together BUP providers and pharmacists. A pilot trial in which the pharmacist handled BUP management including adjustments for withdrawal, in consultation with the provider, found that this model significantly boosts retention in treatment compared to usual provider-based care.11
Budget Policy: The FY 2025 President’s Budget request is $391.1 million, a decrease of $1.6 million compared with the FY 2023 Final level.
The Division of Therapeutics and Medical Consequences
The Division of Therapeutics and Medical Consequences (DTMC) supports research to evaluate the safety and efficacy of pharmacotherapies, behavioral interventions, and medical devices to prevent and treat SUD and drug overdose. This work spans all phases of medical product development including synthesis and preclinical evaluation of potential therapeutics, clinical trial design and execution, and preparing regulatory submissions.
DTMC supports research on a diverse array of pharmacotherapies, including repurposing of drugs used for other conditions and developing new compounds with novel molecular targets. For example, diabetes drugs like semaglutide, which are based on the hormone glucagon-like peptide-1 (GLP-1), may hold potential for treating SUD. GLP-1 analogs help control food cravings, and anecdotal reports from people taking these drugs for diabetes or weight loss suggest they might help reduce drug cravings, too. NIDA is supporting preclinical studies of GLP-1 analogs for alcohol and opioid addiction and a small clinical trial for smoking cessation. While stimulants generally work by increasing dopamine signals in the brain, methamphetamine and cocaine use disorder (MtUD and CcUD) also involve changes in glutamate signaling. A phase I clinical trial is underway to investigate treating CcUD with a small-molecule inhibitor of the glutamate receptor mGluR5.
DTMC also supports research on therapies involving psychedelic and dissociative drugs. For example, a large randomized controlled trial is investigating whether psilocybin in combination with psychotherapy can improve smoking cessation. Other trials are investigating ketamine-assisted therapy for CcUD and MtUD, and as a bridge to start MOUD. (For more, see “Accelerating therapeutics development for drug addiction and overdose.” below)
Accelerating therapeutics development for drug addiction and overdose
Additional treatments for substance use disorders (SUD) are urgently needed. Although there are effective medications for opioid, tobacco, and alcohol use disorders, they do not work for everyone. Moreover, no proven-effective medications exist for other SUDs. To address these gaps, NIDA has made significant investments in the following areas of drug development:
New formulations of medications for opioid use disorder (MOUD) are under study, including oral methadone formulations designed to last longer by resisting stomach acids, and devices that provide longer-lasting drug delivery, like transdermal naltrexone patches and subdermal nalmefene implants.
Vaccines for SUD contain small molecules that structurally mimic a drug. If the drug is taken, the vaccine generates antibodies that stick to it and prevent it from entering the brain. Anti-cocaine and anti-opioid vaccines are currently in human testing.
Monoclonal antibody therapies, which bypass the vaccination step but work on the same principle, are also under study.
Sequestrants—small molecules designed to trap drugs and clear them from the body—are under development to reverse opioid overdose and methamphetamine intoxication.
Repurposing drugs, like the diabetes drug semaglutide, is another active area. NIDA intramural researchers recently found that semaglutide reduces binge-link drinking and alcohol dependence in rodent models.
Respiratory stimulants are intended to reverse overdose by activating critical brain pathways to restore breathing, instead of blocking the drug. Some respiratory stimulants activate the carotid bodies, sensory organs in the carotid arteries, alerting the brain to low oxygen levels in the blood.
New compounds with novel targets include small molecules that target nociceptin receptors. Nociceptin is a small protein named for its role in perceiving pain (nociception), and its receptor is closely related to the mu opioid receptor. The nociceptin system was discovered less than three decades ago through NIDA-funded research, and there is still much to learn about its potential roles in treating pain and addiction.
Another focus of DTMC’s portfolio is neuromodulation, which involves stimulating the brain to reset the circuitry underlying brain disorders. Deep brain stimulation— which involves inserting electrodes into the brain and is approved by the Food and Drug Administration (FDA) for treating Parkinson’s disease and epilepsy—is in clinical trials for refractory OUD. DTMC also supports research on non-invasive neuromodulation therapies, including low-intensity focused ultrasound and transcranial magnetic stimulation (TMS). TMS is FDA-approved as an adjunct therapy for smoking cessation, in part due to NIDA-funded research, and is now being investigated for other SUDs.
DTMC has a growing portfolio of research to address OUD and other co-occurring mental health disorders. For example, 40-60 percent of people with OUD suffer from chronic pain and depression, which can increase their risk of opioid misuse. To improve health outcomes for these patients, researchers are evaluating an approach in which primary providers and behavioral health specialists provide collaborative care. In FY 2023, DTMC announced new funding to develop pharmacotherapies, behavioral therapies, and devices for co-occurring OUD and mental illness.
Budget Policy: The FY 2025 President’s Budget request is $109.8 million, a decrease of $0.5 million compared with the FY 2023 Final level.
The Center for Clinical Trials Network
The Center for Clinical Trials Network (CCTN) manages NIDA’s National Drug Abuse Treatment Clinical Trials Network (CTN), which provides a collaborative framework for healthcare providers, researchers, and patients to conduct clinical trials on the safety and efficacy of SUD interventions. The CTN includes 16 research nodes and more than 240 community-anchored treatment programs across the country. This unique structure enables clinical research on behavioral, pharmacological, and integrated therapies across diverse settings and populations, and implementation studies that help bring research results into practice. Active CTN studies are exploring primary prevention of SUD; increasing patient access and adherence to MOUD, especially in rural and underserved populations; evaluating potential medications for stimulant use disorder; and addressing stigma and other barriers to SUD treatment.
Since adolescence is a critical time for susceptibility to SUD and responsiveness to interventions, the CTN prioritizes development and testing of screening tools tailored to this age range. A recent study tested three brief SUD screening tools in a large, diverse adolescent population and found that they performed well compared to an in-depth diagnostic interview.12 Through the ongoing Subthreshold OUD Prevention (STOP) trial, the CTN seeks to determine if screening and intervention for low-severity OUD in primary care can reduce OUD progression and overdose risk.
The CTN is also testing strategies to identify OUD patients and start MOUD treatment in emergency departments (EDs)—at the frontline of the overdose epidemic. In one approach, the CTN designated champions to educate ED and community clinicians on BUP treatment and how to overcome stigma and other barriers to its use. Implementing this approach for six months led to higher rates of standard oral BUP initiation in the ED and referral to ongoing OUD treatment in the community, and was feasible even in rural and low-resource EDs.14
The CTN is developing and evaluating potential treatments for MtUD and CcUD, for which there are no FDA-approved therapies. A recent CTN trial found that a combination of injectable naltrexone and oral bupropion, a commonly prescribed medication for depression and nicotine cessation, was effective in helping people with MtUD reduce their use. A secondary analysis focused on men who have sex with men—who are at higher risk for MtUD and harmful outcomes, including HIV infection—and found they had higher response rates to this treatment than heterosexual men.15 Two other CTN trials will examine whether patients with MtUD or CcUD and opioid co-use will benefit from injectable naltrexone and monthly injectable BUP.
Budget Policy: The FY 2025 President’s Budget request is $35.7 million, a decrease of $0.1 million compared with the FY 2023 Final level.
Pioneering research on wastewater testing to detect drugs and infectious diseases
Turning the tide of the overdose epidemic requires faster, more comprehensive measures of community drug use patterns. Traditional measures such as ED visits and SUD treatment admissions have a significant lag time and typically only capture people who seek healthcare for their drug use. Wastewater-based epidemiology (WBE), an approach for measuring drugs and other substances in sewage wastewater, can provide data in near real-time from the community at large. NIDA has provided seminal and ongoing support for WBE to monitor drug threats and even helped pioneer its use to monitor SARS-CoV-2, the virus that causes COVID-19.
NIDA supported one of the earliest studies of WBE-based drug monitoring in the United States, published in 2009, which found that it can complement more traditional methods. Within a decade, NIDA began supporting several projects to improve the utility of WBE for drug monitoring, including the development of an automated robotic system called Biobot to test wastewater at manholes.
This research helped provide a foundation for applying WBE to monitor the spread of SARS-CoV-2. NIH-funded studies showed that wastewater detection of the virus precedes clinical case reports by several days, and the Centers for Disease Control and Prevention continues to operate a national WBE system to provide early warnings of COVID-19 surges.
NIDA is now supporting research to implement WBE-based drug monitoring on a larger scale. For example, NIDA’s National Drug Early Warning System (NDEWS) uses and evaluates methods to provide real-time surveillance of potential drug threats. In FY 2021 and FY 2023, NIDA provided additional funding for NDEWS to invest in WBE and integrate its data with other drug data streams. NIDA is also funding Biobot to conduct a yearlong analysis of community fluctuations in drug use across the United States.
The Office of Translational Initiatives and Program Innovations
The Office of Translational Initiatives and Program Innovations (OTIPI) translates discoveries in addiction research into candidate health applications. OTIPI supports translational research through NIDA’s Small Business Innovation Research/Technology Transfer (SBIR/STTR) programs, as well as Challenge competitions. OTIPI also develops training programs that help scientists move their discoveries from the lab to the real world.
In FY 2023, OTIPI issued a Primary Care Challenge competition to propose models for how primary care providers can more effectively identify people at risk for substance use and deliver interventions to reduce this risk. Three winning projects were selected. One project will focus on recently incarcerated adults, who are at high risk for drug use and overdose, with community health workers leading patient outreach, substance use screening, and linkages to preventive care. Another project will screen teens with a mental health diagnosis for substance use risk, and the third will use artificial intelligence (AI) to identify high-risk pre-teens based on their electronic health records.
OTIPI supports medical device development, including devices to treat neonatal opioid withdrawal syndrome (NOWS), which can affect infants exposed to opioids in the womb. For example, an SBIR project has developed a vibrating crib mattress to soothe infants with NOWS. In a large trial, infants who slept on this mattress needed less medication to manage withdrawal symptoms and left the hospital three days earlier on average than infants who received only usual care.16 Another SBIR project focuses on treating NOWS by retooling an FDA-approved technology for opioid withdrawal in adults, called transcutaneous auricular neurostimulation. This technology uses an earpiece to painlessly stimulate nerves near the ears, transmitting signals that are believed to cause release of the brain’s own endogenous opioids and help control withdrawal. A safety trial found that this technology was well-tolerated by infants with NOWS, and efficacy trials are planned.17
OTIPI also supports development of SUD treatment modalities that are powered by AI. One example is Woebot, a conversational smartphone app originally designed to help people struggling with depression. SBIR-funded researchers have modified Woebot to help people with SUD track their mood and drug cravings, and to provide coaching in behavior change. In a pilot study, after using Woebot-SUD for eight weeks, participants reported increased ability to resist craving and had improved scores on SUD screening tests.18 Results from a larger trial are expected soon. Other researchers are working on a wrist-worn device for MOUD patients that would record biometric data, including mobility and skin temperature; use AI to read those data for signs of opioid withdrawal or relapse; and alert providers so that they can adjust treatment.
OTIPI is also supporting the development of tools to remove barriers to methadone treatment for OUD, which is available only from federally regulated opioid treatment programs (OTPs). Continuing flexibilities implemented during the COVID-19 pandemic allow OTPs to dispense up to 28 days of take-home methadone for stable patients, but clinicians have significant discretion—and difficult decisions—regarding take-home doses for less stable patients. In a pilot study, an OTP in Washington enabled patients to submit video confirmation that they were using their take-home methadone as prescribed. Compared to regular OTP clients, pilot participants had more days of observed dosing and were more often approved for increased take-home doses.19 Other researchers are developing a wearable biosensor to remotely monitor patients’ adherence to their take-home methadone, and a new OTIPI-led program calls for further research on low-cost point-of-need approaches to lower barriers to SUD care.
Budget Policy: The FY 2025 President’s Budget request is $43.2 million, a decrease of $0.2 million compared with the FY 2023 Final level.
The NIH HEAL Initiative
The NIH HEAL Initiative, which is co-led by NIDA and the National Institute of Neurological Disorders and Stroke (NINDS), aims to accelerate scientific solutions to the opioid crisis. One of its major focus areas has been research on how to implement effective prevention and treatment interventions for OUD and overdose across healthcare and non-healthcare settings. The HEALing Communities Study (HCS), led by NIDA in collaboration with the Substance Abuse and Mental Health Services Administration (SAMHSA), is testing the impact of integrating evidence-based interventions for OUD and overdose in 67 communities spanning 4 states. HCS communities have deployed over 1,000 evidence-based strategies to expand access to MOUD, implement overdose education and naloxone distribution, and reduce high risk prescribing. Lessons learned from the HCS have been captured in two practice guides to help providers, public health agencies, and others assemble community coalitions and implement effective approaches to reduce overdose deaths.
The Justice Community Opioid Innovation Network (JCOIN) is studying approaches to improve evidence-based treatment for people with OUD in criminal-legal settings. JCOIN has found that MOUD access during incarceration not only saves lives but is also associated with 32 percent lower recidivism after release.20 JCOIN recently disseminated a first-of-its-kind MOUD Budget Impact tool to helps jail and prison administrators estimate the cost of providing MOUD services.21 NIDA plans to extend JCOIN through at least FY 2030, with a focus on research to scale up MOUD and other evidence-based interventions across justice settings and where they intersect with community-based treatment.
The HEAL Harm Reduction Research Network is developing and testing strategies to prevent overdose, transmission of HIV and hepatitis C virus, and other harms associated with drug use. Current projects are studying delivery of harm reduction services during emergency care, and via mobile vans and smartphones for hard-to-reach patients. The network is also examining the impact of state and local harm reduction policies. In New York and Rhode Island, researchers are conducting an observational study to examine outcomes associated with overdose prevention centers, which allow people to consume pre-obtained drugs under the supervision of staff who are trained in addiction and overdose treatment.
With HEAL funding, NIDA’s Recovery Research Networks are working to build an evidence base for effective recovery support services. While such services have strong foundations in the lived experiences of people with SUD, most have not been formally evaluated. These networks are engaging recovery service providers and people with lived experience in research to examine what types of recovery services work best for different people and to help link recovery services with established standards of care including MOUD.
A new HEAL program funded in FY 2023—Research to Foster an OUD Treatment System Patients Can Count On—is supporting four projects in which researchers are working with providers, patients, payors, and public health agencies to develop quality measures to improve patient outcomes in OTPs and other settings.
Budget Policy: The FY 2025 President’s Budget request is $355.3 million, which is equal to the FY 2023 Final level. This includes $12.8 million for Research Management and Support related to this area of research.
The NIDA Intramural Research Program
The NIDA Intramural Research Program (IRP) conducts research to inform strategies for prevention and treatment of SUD and related health outcomes. The IRP portfolio includes research to elucidate the mechanisms underlying SUDs, evaluate potential new therapies, and identify and characterize emerging drugs such as synthetic opioids, stimulants, and cannabinoids.
IRP researchers who study how opioids affect brain and respiratory functions were able to pivot quickly to study the additive effects of xylazine. In preclinical studies, they found that fentanyl exposure causes respiratory suppression, which produces a rapid, robust decrease in oxygen flow to the brain, followed by a gradual rebound. Adding xylazine to fentanyl eliminated this rebound, prolonging the brain’s oxygen deficit—which could contribute to the increasing involvement of xylazine in opioid overdose deaths.22
The IRP Designer Drug Research Unit focuses on emerging synthetic drugs with addiction potential, including stimulants called synthetic cathinones. Like other stimulants, cathinones increase dopamine levels at synapses, sometimes by blocking the activity of transporters that clean up excess dopamine. Illicit synthetic cathinones, also called “bath salts,” can be extremely potent, with longer lasting effects than cocaine. IRP researchers found that this is due to unusually long-lasting inhibition of dopamine transporters.23 These researchers are also investigating cathinone derivatives that act preferentially on serotonin transporters as an alternative to selective serotonin reuptake inhibitors (like Prozac) for depression and anxiety.24
IRP researchers also are examining the neurobiological basis for opioid withdrawal symptoms, which contribute to relapse risk. One recent study investigated brain pathways responsible for increased pain sensitivity (or hyperalgesia) during opioid withdrawal. Through a combination of brain imaging and chemogenetics—the use of designer drugs and receptors to control neuronal activity—researchers identified a cell type in the mouse brainstem that contributes to hyperalgesia (see cover image).25 Future neuromodulation therapies or other approaches could target those cells to reduce hyperalgesia and relapse risk.
Other IRP researchers are studying the addiction potential of S-ketamine. While S-ketamine is FDA-approved for treating severe depression, it has addiction potential that is thought to occur through opioid signaling. IRP researchers with expertise in functional brain imaging partnered with other NIH researchers to investigate this idea. They found that in rats, S-ketamine acts on opioid receptors to stimulate activity in the nucleus accumbens—a brain region associated with addiction—and that self-administration of S-ketamine leads to opioid tolerance.26 Those findings have implications for monitoring the safety of S-ketamine therapy—both its current use for depression and emergent uses for opioid and stimulant addiction.
Budget Policy: The FY 2025 President’s Budget request is $120.9 million, an increase of $4.3 million or 3.7 percent compared with the FY 2023 Final level.
Research Management and Support
Research Management and Support (RMS) activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Staff supported by NIDA’s RMS budget also coordinate training and career development programs to sustain a talented, diverse workforce of addiction scientists. Other RMS functions include strategic planning, coordination, dissemination of research findings and funding opportunities, program evaluation, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA RMS funding also supports evidence-based education and outreach about substance use and addiction to inform health policy and practice and provide the public with timely, accessible, trustworthy information about drug research in English and Spanish.
Budget Policy: The FY 2025 President’s Budget request is $92.9 million, an increase of $7.3 million or 8.6 percent compared with the FY 2023 Final level.
References
- https://pubmed.ncbi.nlm.nih.gov/37250466
- https://pubmed.ncbi.nlm.nih.gov/35320704
- https://pubmed.ncbi.nlm.nih.gov/36525955
- https://pubmed.ncbi.nlm.nih.gov/35347109
- https://pubmed.ncbi.nlm.nih.gov/37072167
- https://pubmed.ncbi.nlm.nih.gov/37198725
- https://pubmed.ncbi.nlm.nih.gov/37582630
- https://pubmed.ncbi.nlm.nih.gov/36722118
- https://pubmed.ncbi.nlm.nih.gov/37130880
- https://pubmed.ncbi.nlm.nih.gov/36906496
- https://pubmed.ncbi.nlm.nih.gov/36630629
- https://pubmed.ncbi.nlm.nih.gov/37213103
- https://pubmed.ncbi.nlm.nih.gov/37017967 ; https://pubmed.ncbi.nlm.nih.gov/37140493
- https://pubmed.ncbi.nlm.nih.gov/36995717
- https://pubmed.ncbi.nlm.nih.gov/33497547 ; https://pubmed.ncbi.nlm.nih.gov/37478502
- https://pubmed.ncbi.nlm.nih.gov/37184872
- https://pubmed.ncbi.nlm.nih.gov/33762918
- https://pubmed.ncbi.nlm.nih.gov/33755028
- https://pubmed.ncbi.nlm.nih.gov/36215911
- https://pubmed.ncbi.nlm.nih.gov/35063323
- https://pubmed.ncbi.nlm.nih.gov/36880906 ; https://www.jcoinctc.org/resources/budget-impact-tool
- https://pubmed.ncbi.nlm.nih.gov/37340247
- https://pubmed.ncbi.nlm.nih.gov/36730201
- https://pubmed.ncbi.nlm.nih.gov/36352123
- https://pubmed.ncbi.nlm.nih.gov/35728954
- https://pubmed.ncbi.nlm.nih.gov/36841701
Download as a PDF - NIDA CJ 2025 (PDF, 5.1 MB). Note - this document is not fully accessible, for any issues, please use the web version above.