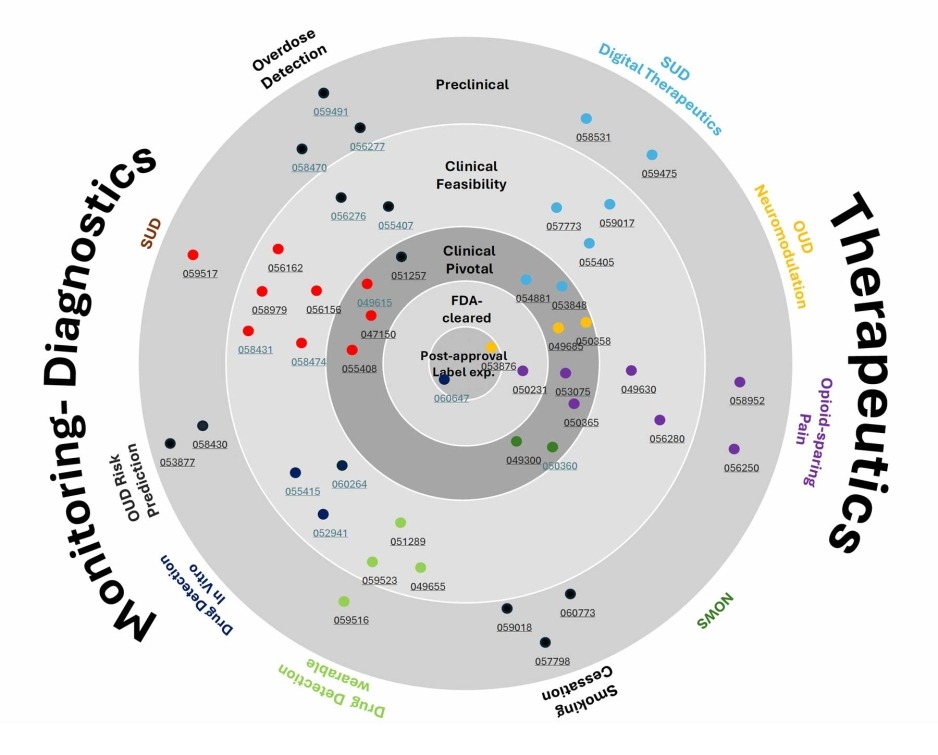
OTIPI SBIR/STTR Therapeutic Devices
- SUD Digital Therapeutics
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - OUD Neuromodulation
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - Opioid-sparing Pain
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - NOWS
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - Smoking Cessation
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion
OTIPI SBIR/STTR Monitoring-Diagnostic Devices
- Overdose Detection
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - SUD
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - OUD Risk Prediction
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - Drug Detection - In-Vitro
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion - Drug Detection - Wearable
Preclinical Clinical Feasibility Clinical Pivotal FDA cleared Post-approval Label expansion