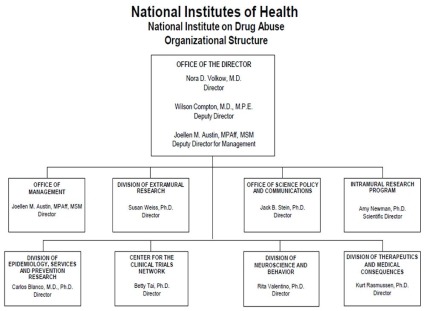
Organizational Chart
- Text Description of Organizational Chart
Office of the Director
- Nora D. Volkow, M.D.
Director - Wilson Compton, M.D., M.P.E.
Deputy Director - Joellen M. Austin, MPAff, MSM
Deputy Director for Management
Office of Management
- Joellen M. Austin, MPAff, MSM
Director
Division of Extramural Research
- Susan Weiss, Ph.D.
Director
Office of Science Policy and Communications
- Jack B. Stein, Ph.D.
Director
Intramural Research Program
- Amy Newman, Ph.D.
Scientific Director
Division of Epidemiology, Services and Prevention Research
- Carlos Blanco, M.D., Ph.D.
Director
Center for the Clinical Trials Network
- Betty Tai, Ph.D.
Director
Division of Neuroscience and Behavior
- Rita Valentino, Ph.D.
Director
Division of Therapeutics and Medical Consequences
- Kurt Rasmussen, Ph.D.
Director
- Nora D. Volkow, M.D.
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to drug abuse, [$1,479,660,000] $1,852,503,000.
Tables
- Budget Mechanism
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2020
FinalFY 2021
EnactedFY 2022
President's
BudgetFY 2022
+/-
FY 2021
EnactedNo. Amount No. Amount No. Amount No. Amount Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Research Projects: Noncompeting 949 $635,899 1,020 $709,701 805 $633,057 -215 -$76,644 Administrative Supplements (113) 12,666 (61) 5,528 (57) 6,000 (-4) 472 Competing: Renewal 47 37,174 66 53,862 69 56,556 3 2,693 New 293 208,458 179 135,821 658 543,374 479 407,553 Supplements 14 2,267 16 2,658 18 3,000 2 342 Subtotal, Competing 354 $247,899 261 $192,341 745 $602,929 484 $410,588 Subtotal, RPGs 1,303 $896,464 1,281 $907,570 1,550 $1,241,986 269 $334,416 SBIR/STTR 92 43,082 86 40,610 114 54,730 28 14,210 Research Project Grants 1,395 $939,546 1,367 $948,180 1,664 $1,296,717 297 $348,537 Research Centers: Specialized / Comprehensive 26 $53,417 26 $53,800 28 $56,252 2 $2,452 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 250 0 200 0 200 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 26 $53,667 26 $54,000 28 $56,452 2 $2,452 Other Research: Research Careers 229 $42,063 229 $42,621 244 $44,725 15 $2,104 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 28 91,126 27 95,244 29 100,244 2 5,000 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 1,270 0 1,311 0 1,311 0 0 Other 92 29,872 100 32,256 112 35,790 12 3,534 Other Research 349 $172,330 356 $171,432 385 $182,070 29 $10,638 Total Research Grants 1,770 $1,165,543 1,749 $1,173,612 2,077 $1,535,239 328 $361,627 Individual Awards 124 $5,615 172 $7,946 172 $8,320 0 $374 Institutional Awards 388 20,871 343 20,054 343 20,743 0 689 Total Research Training 512 $26,486 515 $28,000 515 $29,063 0 $1,063 Research and Development Contracts 93 $89,653 93 $99,623 93 $104,739 0 $5,116 (SBIR/STTR) (non-add) (5) (3,601) (4) (6,695) (4) (6,495) (0) (-200) -Intramural Research 117 100,748 121 102,098 121 105,161 0 3,063 Res. Management & Support 246 75,293 267 76,975 267 78,301 0 1,326 Res. Management & Support (SBIR Admin) (non-add) (0) (311) (0) (274) (0) (274) (0) (0) Construction 0 0 0 0 0 0 0 0 Buildings and Facilities 0 0 0 0 0 0 0 0 Total, NIDA 363 $1,457,724 388 $1,480,309 388 $1,852,503 0 $372,194 1 All numbers in italics and brackets are non-add entries.
- Amounts Available for Obligation
(Dollars in Thousands)1 Source of Funding FY 2020
FinalFY 2021
EnactedFY 2022
President's
BudgetAppropriation $1,462,016 $1,479,660 $1,852,503 Secretary's Transfer 0 0 0 OAR HIV/AIDS Transfers -4,292 649 0 Subtotal, adjusted budget authority $1,457,724 $1,480,309 $1,852,503 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $1,457,724 $1,480,309 $1,852,503 Unobligated balance lapsing -41 0 0 Total obligations $1,457,683 $1,480,309 $1,852,503 1Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2020 - $79,270 FY 2021 - $112,158 FY 2022 - $112,557- Summary of Changes
(Dollars in Thousands) FY 2021 Enacted $1,480,309 FY 2022 President's Budget $1,852,503 Net change $372,194 FY 2021 Enacted FY 2022 President's Budget IC Adjustment Amount Built-In Change from
FY 2021 Enacted Budget AuthorityCHANGES FTEs Budget Authority FTEs Budget Authority Budget Authority FTEs Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of January 2021 pay increase & benefits $29,162 $30,023 $0 $81 b. January FY 2022 pay increase & benefits 29,162 30,023 0 781 c. Paid days adjustment 29,162 30,023 0 0 d. Differences attributable to change in FTE 29,162 30,023 0 0 e. Payment for centrally furnished services 12,481 113,105 0 624 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs60,456 62,033 0 1,679 Subtotal $0 $3,165 2. Research Management and Support: a. Annualization of January 2021 pay
increase & benefits$40,268 $41,493 $0 $109 b. January FY 2022 pay increase & benefits 40,268 41,493 0 1,117 c. Paid days adjustment 40,268 41,493 0 0 d. Differences attributable to change in FTE 40,268 41,493 0 0 e. Payment for centrally furnished services 6,221 6,032 0 -189 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs30,486 30,775 0 853 Subtotal $0 $1,890 Subtotal, Built-in $0 $5,5054 Summary of Changes - Continued
(Dollars in Thousands)FY 2021 Enacted FY 2022 President's Budget IC Adjustment Amount Built-In Change from
FY 2021 Enacted Budget AuthorityCHANGES No. Amount No. Amount Amount No. Amount B. Program: 1. Research Project Grants: a. Noncompeting 1020 $715,229 735 $639,057 -285 -$76,172 b. Competing 261 192,341 745 602,929 484 410,588 c. SBIR/STTR 86 40,610 114 54,730 28 14,120 Subtotal, RPGs 1,367 $948,180 1,594 $1,296,717 $0 227 $348,537 2. Research Centers 26 $54,000 28 $56,452 2 $2,452 3. Other Research 356 171,432 385 182,070 29 10,638 4. Research Training 515 28,000 515 29,063 0 1,063 5. Research and development contracts 93 99,623 93 104,739 0 5,116 Subtotal, Extramural $1,301,236 $1,669,041 $0 $367,805 FTEs FTEs FTEs 6. Intramural Research 121 $102,098 121 $105,161 $0 0 -$102 7. Research Management and Support 267 76,975 267 78,301 0 0 -564 8. Construction 0 0 0 0 9. Buildings and Facilities 0 0 0 0 Subtotal, program 388 $1,480,309 388 $1,852,503 $0 0 $367,140 Total built-in and program changes $0 $372,194 - Authorizing Legislation
PHS Act/Other Citation U.S. Code Citation 2021 Amount Author-
izedFY 2021 Enacted 2022 Amount Author
-izedFY 2022 President's Budget Research and Investigation Section 301 42§241 Indefinite $1,480,309,000 Indefinite $1,852,503,00 National Institute on Drug Abuse Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,480,309,000 $1,852,503,00 - Appropriations History
Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2013 $1,054,001,000 $1,057,196,000 $1,053,367,366 Rescission $2,106,735 Sequestration ($52,871,798) 2014 $1,071,612,000 $1,064,490,000 $1,025,435,000 Rescission $0 2015 $1,023,268,000 $1,028,614,000 Rescission $0 2016 $1,047,397,000 $1,050,875,000 $1,069,086,000 $1,077,488,000 Rescission $0 20171 $1,050,550,000 $1,107,700,000 $1,103,032,000 1,090,853,000 Rescission $0 2018 $864,998,000 $1,107,497,000 $1,113,442,000 $1,383,603,000 Rescission $0 2019 $1,137,403,000 $1,400,126,000 $1,420,591,000 $1,419,844,000 Rescission $0 2020 $1,296,379,000 $1,489,237,000 $1,490,498,000 $1,462,016,000 Rescission $0 2021 $1,431,770,000 $1,476,590,00 $1,505,192,000 $1,479,660,000 Recession $0 2022 $1,852,503,000 1Budget Estimate to Congress includes mandatory financing.
- Budget Authority by Activity
(Dollars in thousands)1 Extramural Research FY 2020
FinalFY 2021
EnactedFY 2022
President's
BudgetFY 2022
+/-
FY 2021 EnactedFTE Amount FTE Amount FTE Amount FTE Amount Detail: Division of Therapeutics and Medical Consequences $114,238 $116,131 $142,295 $26,164 Division of Neuroscience and Behavior 480,779 492,754 603,769 111,015 Division of Epidemiology, Services and Prevention Research 335,590 341,150 418,009 76,859 Center for the Clinical Trials Network 39,001 39,647 48,580 8,932 Office of Translational Initiatives and Program Innovations 48,859 45,659 55,945 10,287 HEAL Initiative2 263,216 265,895 400,443 134,548 Subtotal, Extramural $1,281,682 $1,301,236 $1,669,041 $367,805 Intramural Research 117 $100,748 121 $102,098 121 $105,161 0 $3,063 Research Management & Support 246 $75,293 267 $76,975 267 $78,301 0 $1,326 TOTAL 363 $1,457,724 388 $1,480,309 388 $1,852,503 0 $372,194 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Total for HEAL Initiative including RMS is (in thousands) $266,321 in FY 2020, $270,295 in FY 2021, and $405,443 in FY 2022.- Budget Authority by Object Class
(Dollars in Thousands)1 FY 2021 Enacted FY 2022 President's Budget FY 2022 +/- FY 2021 Enacted OBJECT CLASSES FY 2021 Enacted FY 2022 President's Budget FY 2022 +/- FY 2021 Total compensable workyears: Full-time equivalent 388 388 0 Full-time equivalent of overtime and holiday hours 0 0 0 Average ES salary $199 $204 $5 Average GM/GS grade 13.0 13.0 0.0 Average GM/GS salary $128 $131 $3 Average salary, Commissioned Corps (42 U.S.C. 207) $122 $125 $3 Average salary of ungraded positions $154 $158 $4 Personnel Compensation: 11.1 Full-time permanent 30,606 31,190 584 11.3 Other than full-time permanent 13,552 13,860 308 11.5 Other personnel compensation 1,614 1,651 37 11.7 Military personnel 836 971 135 11.8 Special personnel services payments 5,840 5,973 133 11.9 Subtotal Personnel Compensation $52,448 $53,645 $1,197 12.1 Civilian personnel benefits 16,528 17,406 878 12.2 Military personnel benefits 453 466 13 13.0 Benefits to former personnel 0 0 0 Subtotal Pay Costs $69,429 $71,517 $2,088 21.0 Travel and transportation of persons 433 690 258 22.0 Transportation of things 235 239 4 23.1 Rental payments to GSA 0 0 0 23.2 Rental payments to others 0 0 0 23.3 Communications, utilities and misc. charges 2,391 2,434 43 24.0 Printing and reproduction 0 0 0 25.1 Consulting services 47,443 46,043 -1,400 25.2 Other services 18,993 19,234 240 25.3 Purchase of goods and services from government accounts 100,893 107,439 6,547 25.4 Operation and maintenance of facilities 519 522 3 25.5 Research and development contracts 23,499 23,922 423 25.6 Medical care 499 518 18 25.7 Operation and maintenance of equipment 6,024 6,133 108 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal Other Contractual Services $197,870 $203,810 $5,940 26.0 Supplies and materials 4,924 5,038 114 31.0 Equipment 4,309 4,386 78 32.0 Land and structures 34 35 1 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions 1,200,683 1,564,353 363,669 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends 1 1 0 44.0 Refunds 0 0 0 Subtotal Non-Pay Costs $1,410,880 $1,780,986 $370,106 Total Budget Authority by Object Class $1,480,309 $1,852,503 $372,194 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses
(Dollars in Thousands) OBJECT CLASSES FY 2021 Enacted FY 2022 President's Budget FY 2022 +/- FY 2021 Personnel Compensation: Full-time permanent (11.1) $30,606 $31,190 $584 Other than full-time permanent (11.3) 13,552 13,860 308 Other personnel compensation (11.5) 1,614 1,651 37 Military personnel (11.7) 836 971 135 Special personnel services payments (11.8) 5,840 5,973 133 Subtotal Personnel Compensation (11.9) $52,448 $53,645 $1,197 Civilian personnel benefits (12.1) $16,528 $17,406 $878 Military personnel benefits (12.2) 453 466 13 Benefits to former personnel (13.0) 0 0 0 Subtotal Pay Costs $69,429 $71,517 $2,088 Travel and transportation of persons (21.0) $433 $690 $258 Transportation of things (22.0) 235 239 4 Rental payments to others (23.2) 0 0 0 Communications, utilities and miscellaneous charges (23.3) 2,391 2,434 43 Printing and reproduction (24.0) 0 0 0 Other Contractual Services: Consultant services (25.1) 38,404 39,197 790 Other services (25.2) 18,993 19,234 240 Purchases from government accounts (25.3) 54,686 57,726 3,040 Operation and maintenance of facilities (25.4) 519 522 3 Operation and maintenance of equipment (25.7) 6,024 6,133 108 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $118,631 $122,812 $4,181 Supplies and materials (26.0) $4,924 $5,038 $114 Subtotal Non-Pay Costs $126,614 $131,213 $4,600 Total Administrative Costs $196,043 $202,730 $6,687 - Details of Full-Time Equivalent Employment (FTEs)
OFFICE/
DIVISIONFY 2020
FinalFY 2021
EnactedFY 2022
President's BudgetCivilian Military Total Civilian Military Total Civilian Military Total Center for the Clinical Trials Network Direct: 13 0 13 14 0 14 14 0 14 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 13 0 13 14 0 14 14 0 14 Division of Epidemiology, Services and Prevention Research Direct: 24 2 26 26 1 27 26 1 27 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 24 2 26 26 1 27 26 1 27 Division of Extramural Research Direct: 44 0 44 44 0 44 44 0 44 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 44 0 44 44 0 44 44 0 44 Division of Neuroscience and Behavior Direct: 24 0 24 25 0 25 25 0 25 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 24 0 24 25 0 25 25 0 25 Division of Therapeutics and Medical Consequences Direct: 28 0 28 29 0 29 29 0 29 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 28 0 28 29 0 29 29 0 29 Intramural Research Program Direct: 112 4 116 116 4 120 116 4 120 Reimbursable: 1 0 1 1 0 1 1 0 1 Total: 113 4 117 117 4 121 117 4 121 Office of Management Direct: 21 0 21 23 0 23 23 0 23 Reimbursable: 49 0 49 60 0 60 60 0 60 Total: 70 0 70 83 0 83 83 0 83 Office of Science Policy and Communication Direct: 23 0 23 22 0 22 22 0 22 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 23 0 23 22 0 22 22 0 22 Office of the Director Direct: 18 0 18 23 0 23 23 0 23 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 18 0 18 23 0 23 23 0 23 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 357 6 363 383 5 388 383 5 388 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2018 13.0 2019 13.3 2020 13.1 2021 13.0 2022 13.0 - Detail of Positions
Detail of Positions1 GRADE FY 2020 Final FY 2021 Enacted FY 2022 President's Budget Total, ES Positions 1 1 1 Total, ES Salary 197,300 199,273 203,856 GM/GS-15 65 61 61 GM/GS-14 66 70 70 GM/GS-13 82 90 90 GS-12 46 44 44 GS-11 11 14 14 GS-10 0 0 0 GS-9 8 9 9 GS-8 5 8 8 GS-7 7 4 4 GS-6 2 2 2 GS-5 0 0 0 GS-4 0 1 1 GS-3 0 0 0 GS-2 0 0 0 GS-1 0 0 0 Subtotal 292 303 303 Commissioned Corps (42 U.S.C. 207): Assistant Surgeon General 0 0 0 Director Grade 3 2 2 Senior Grade 2 2 2 Full Grade 1 1 1 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 6 5 5 Ungraded 91 93 93 Total permanent positions 297 309 309 Total positions, end of year 390 402 402 Total full-time equivalent (FTE) employment, end of year 363 388 388 Average ES salary 197,300 199,273 203,856 Average GM/GS grade 13.1 13.0 13.0 Average GM/GS salary 126,642 127,908 130,850 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Budget Graphs
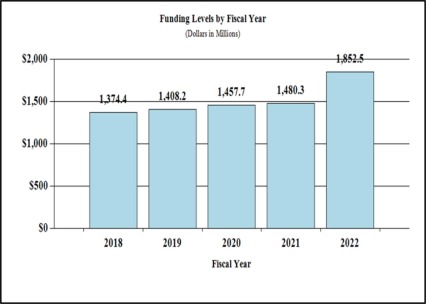
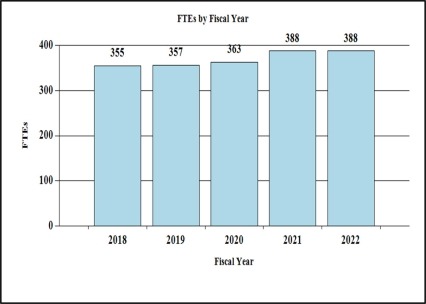
History of Budget Authority and FTEs:
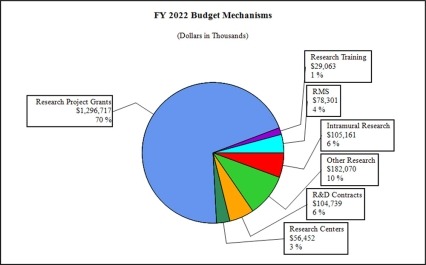
Distribution by Mechanism (dollars in thousands):
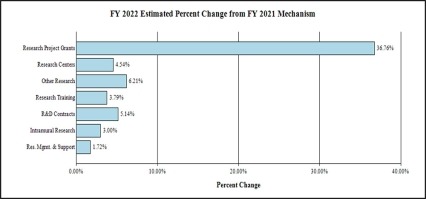
Change by Selected Mechanism:
Justification of Budget Request
National Institute on Drug Abuse
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 | |
|---|---|---|---|---|
| BA | $1,457,724,000 | $1,480,309,000 | $1,852,503,000 | +372,194,000 |
| FTE | 363 | 388 | 388 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Major Changes in the Fiscal Year 2022 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2022 President’s Budget. The FY 2022 President’s Budget for NIDA is $1,852.5 million, an increase of $372.2 million above the FY 2021 enacted level.
- Research Project Grants (RPGs) (+$348.5 million; total $1,296.7 million):
NIDA will significantly increase funding for competing RPGs in support of the additional funding requested for the HEAL Initiative and related research into opioids and pain management. The number of noncompeting RPGs will decrease by 215 in FY 2022 as previously awarded projects complete their performance period, but the number of competing RPGs is expected to increase by over 480 in comparison to the FY 2021 level of 261 awards. The amount of support to competing awards will be increased by $410.6 million from FY 2021, a 213 percent increase.
- Research Centers (+$2.5 million; total $56.5 million): NIDA will increase support to new specialized/comprehensive centers projects.
- Other Research (+$10.6 million; total $182.1 million): NIDA will increase support to research career development awards, research training awards, and cooperative clinical research.
- Ruth L Kirchstein Training (+$1.1 million; total $29.1 million): NIDA will maintain a level of 515 trainees in FY 2022, unchanged from FY 2021.
Director's Overview
The National Institute on Drug Abuse (NIDA) is the lead federal agency supporting scientific research on drug use and its consequences. Its mission is to advance science on drug use and addiction and apply that knowledge to improve individual and public health. After decades of research, addiction is now understood to be a chronic, treatable brain disorder from which one can recover. NIDA-supported research has led to the development of effective prevention and treatment interventions, providing hope for the more than 20 million people in the United States diagnosed with substance use disorders and their loved ones. Although significant strides have been made, there is more work to be done. New and improved interventions and effective strategies for implementing them will be essential to combatting this evolving public health crisis.
NIDA Research Responds to Urgent Public Health Needs
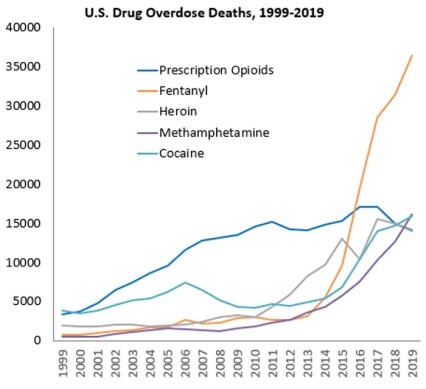
While drug overdoses in the United States have been increasing exponentially for at least 40 years, different substances have driven this increase over time. The 21st century has been marked by overdoses involving opioids. Data from the Centers for Disease Control and Prevention (CDC) show that drug overdose deaths reached a record high in 2019; of nearly 71,000 overdose deaths, over 70 percent involved opioids (see Figure 1).1 The crisis was initially driven by prescription opioids, and later by heroin use. However, since 2016, synthetic opioids, such as fentanyl, account for the largest fraction of overdose deaths. The National Institutes of Health (NIH) Helping to End Addiction Long-termSM (HEAL) Initiative is an aggressive, trans-agency effort to speed scientific solutions to stem the national opioid public health crisis. Within HEAL, NIDA leads numerous programs aimed at treating opioid use disorder (OUD) and reducing overdose mortality.
Stimulants have also emerged as an overdose threat. From 2012 through 2019, the number of deaths involving methamphetamine increased more than 6-fold (from around 2,600 to more than 16,100); the number involving cocaine more than tripled (from around 4,400 to nearly 16,000) (see Figure 1).2 Given the urgent need to confront these dramatic increases, NIDA has prioritized the development of medications to treat stimulant use disorders.
The collision of the overdose crisis with the coronavirus disease 2019 (COVID-19) pandemic puts people with substance use disorders (SUD) at particular risk. Early data show increases in drug use and overdose since the pandemic began,3 and the highest number of overdose deaths (over 83,000) ever recorded occurred in the 12 months ending in June 2020.4 Individuals with SUD, especially Blacks/African Americans and those with OUD, are at higher risk for COVID-19 and its adverse outcomes.5 NIDA is supporting dozens of studies on the intersection of SUD and COVID-19. (See Program Portrait “Intersection of COVID-19 and Substance Use Disorders.”)
Recently, there has been a dramatic increase in vaping cannabinoids and nicotine, particularly among teens and young adults. Nicotine vaping among 12th graders increased from 27.8 percent in 2017 to 37.3 percent in 2018, which is the largest one-year increase for any substance reported in the 45-year history of the Monitoring the Future (MTF) study, an annual NIDA-supported survey that assesses substance use behaviors and attitudes in adolescents and young adults. The second largest increase occurred for marijuana vaping among 12th graders, increasing from 7.5 percent in 2018 to 14 percent in 2019.6,7 NIDA continues to monitor and analyze these and other trends through its portfolio of epidemiology research. Data from MTF and NIDA’s Population Assessment of Tobacco and Health (PATH) study, which assesses patterns of tobacco and nicotine use, showed that flavored e-cigarette products particularly appeal to youth8,9 and informed a 2020 U.S. Food and Drug Administration (FDA) policy prioritizing enforcement against certain unauthorized flavored cartridge-based products that appeal to youth.10
The National Drug Early Warning System (NDEWS), launched in 2014, allows NIDA to monitor patterns of drug use across the nation and rapidly recruit research resources to study them. NDEWS was able to expand in its second iteration in April 2020, incorporating real-time surveillance to detect early signals of emerging crises. The new system, which includes 18 sentinel sites and a coordinating center, uses novel surveillance methods and rapidly harmonizes and disseminates data. NDEWS is currently harnessing its network to collect data on substance use-related consequences of COVID-19, including data from novel informants such as funeral directors, emergency medical service personnel, and syringe exchange service workers.
Addressing Health Disparities and Diversifying the Workforce
Disparities in access to SUD treatment exacerbate the negative impact of addiction on minority populations and individuals in under-resourced settings.11 For example, people of color who are charged with drug-related crimes are more likely to go to prison than White individuals, charged with similar crimes.12 NIDA is investing in research to develop and test interventions that can help address these disparities. Through HEAL, the Justice Community Opioid Innovation Network (JCOIN)13 supported 10 grants and $22 million in FY 2020 to test strategies to increase high quality OUD treatment in legal settings. To address disparities among African American/Black communities in SUD treatment initiation and completion, NIDA supports research on culturally-sensitive intervention strategies. NIDA’s Intervention Research to Improve Native American (NA) Health program focuses on health-promotion and disease-prevention interventions tailored to the needs of NA populations. NIDA’s broad health disparities portfolio also targets treatment disparities for SUD and infectious conditions in sexual and gender minorities and addresses challenges associated with preventing and treating OUD in rural areas. Several approaches leverage technology, including machine learning approaches to analyze electronic health record data to inform personalized, culturally-appropriate treatment.
Scientists and trainees from diverse backgrounds bring different perspectives, creativity, and individual enterprise to address complex scientific problems. Recognizing that the research enterprise requires superior intellect, creativity, and a wide range of skill sets and viewpoints, NIDA participates in the NIH diversity supplement program and supports the NIDA Diversity Scholars Network aimed at improving the funding success of early-stage investigators from underrepresented minority populations. NIDA is also developing an Action Plan to Promote Racial Equity, consisting of specific strategies to ameliorate racial discrimination in the NIDA workplace, increase diversity in NIDA’s scientific workforce, and fill gaps in research related to minority health, health disparities, and the health effects of racism on SUD.
Building on Basic Science to Advance Prevention, Treatment, and Recovery
Decades of research have demonstrated the complex social and biological factors that contribute to substance misuse and addiction, including the long-lasting effects that addictive drugs have on the brain. Advances in genetics, neuroscience, and behavioral science, made possible through NIDA-supported research, have illuminated the factors that influence drug use, how individuals develop SUDs, and how those disorders manifest over time. NIDA supports research to translate findings from basic and epidemiological research into prevention and treatment interventions. For example, studying how opioids interact with their neural receptors led to the development of drugs like naloxone, which can reverse overdoses and save lives, and which researchers further refined into an easy-to-use, quick acting nasal formulation in 2018. Similarly, studying role of the noradrenergic system in stress reactivity informed the development of the adrenergic drug Lofexidine, a medication that specifically targets the physiological symptoms of opioid withdrawal. Lofexidine was developed with NIDA support and approved by the FDA in 2018.
The NIH Helping to End Addiction Long-termSM (HEAL) Initiative
In FY 2018, NIH launched the HEAL InitiativeSM, a trans-agency research effort to improve the prevention and treatment of opioid misuse and addiction and enhance pain management. As part of HEAL, NIDA supported over $250 million annually focused on: translating research into practice for the treatment of opioid addiction in healthcare and legal settings; developing new strategies to prevent and treat opioid addiction; enhancing outcomes for infants and children exposed to opioids; and developing novel medications for OUD and overdose.
Under HEAL, NIH is poised to launch the HEALthy Brain and Child Development (HBCD) Study, which will be the most comprehensive study of early brain development ever conducted.14 Led by NIDA, this study will enroll 7,500 pregnant and postpartum women and follow them and their children from the prenatal period through early childhood (age 9-10) to determine how diverse factors, including maternal drug exposures, influence brain and child development. The study, which will be conducted at multiple research sites across the country and in diverse ethnic and socioeconomic backgrounds, is expected to begin enrollment in 2022, and is currently soliciting applications for study sites, a data coordinating center, and an administrative core.
There is also a vital need to develop preventive strategies that can decrease the incidence and prevalence of opioid misuse and OUD during key periods of vulnerability. NIDA is administering nine studies to develop and test strategies to prevent opioid misuse and OUD among high-risk adolescents and young adults (ages 16-30), which is the age group at highest risk for opioid initiation, misuse, OUD, and overdose fatalities. The studies target this high-risk group in primary care, school-based health centers,15 youth treatment centers, and legal system settings16 in areas most affected by the opioid crisis or with indicators of an emerging crisis.
While effective medications exist for OUD, these medications are underutilized. Suboptimal patient retention in treatment regimens, policy barriers that limit opioid prescribing, and stigma around opioid agonist medications all contribute to their underutilization. More options are needed to help people with OUD achieve long-term recovery. NIDA administered over 40 HEAL grants in FY 2020 focused on medications development research for OUD and overdose. Since HEAL began, 16 Investigational New Drug applications were filed with the FDA and authorized to for human studies. These studies focus on a variety of drug targets, as well as vaccines that could prevent opioids from entering the brain. Others are repurposing existing medications for OUD indications, such as the FDA-approved insomnia medication, suvorexant, based on known overlaps between brain signaling systems involved in sleep and addiction.
To help commercialize the results of NIDA research, the Institute supports translational initiatives to turn research into products for SUD and related indications or attract companies with potentially useful approaches to expand into this traditionally underserved space. Products under development in these programs help deliver counseling via mobile devices, use virtual reality to reduce the need for opioid pain relief, identify the signs of overdose to automatically alert first responders, and provide automated gentle, soothing stimulation to babies born in opioid withdrawal. (See Program Portrait “NIDA’s Innovative Technology Program.”)
NIDA-supported research has revolutionized our understanding of the biological, social, environmental, and systems-level factors that confer risk for or resilience against development of SUDs, and leveraged that understanding to advance prevention, treatment, and recovery. Continued support, both for NIDA and HEAL, will be crucial to realizing the promise of the public investment in addiction research and to addressing this deadly public health crisis. Overall Budget Policy: The FY 2022 President’s Budget request is $1,852.5 million, an increase of $372.2 million or 25.1 percent above the FY 2021 Enacted level. Within this funding level, funding for the HEAL Initiative will increase by $135.1 million or 50.0 percent above the FY 2021 Enacted level. In addition, funding for research into opioids and pain management outside the HEAL Initiative will increase by an additional $196.3 million.
Program Descriptions
Neuroscience and Behavior Research
NIDA’s Division of Neuroscience and Behavior (DNB) advances knowledge of the basic biological mechanisms that underlie drug use and guide the development of novel prevention strategies and treatments for SUD. This includes identifying the effects of illicit substances on brain structure and function throughout the lifespan and across stages of drug use and SUD. Areas of support include studies to identify genetic variants and epigenetic modifications that influence vulnerability to SUD, the effects of drugs on gene expression and brain development and function; the interaction of genes with environmental conditions, including how they influence brain development; and basic processes underlying vulnerability and resilience to SUD. DNB supports research to elucidate the pharmacology of drugs and to leverage this knowledge towards the development of therapeutics to treat SUD, the adverse consequences of illicit drugs, and pain. One recent DNB-supported study found that prenatal exposure to cannabinoids altered the ways the brains of male, but not female, adolescent rats respond to cannabis, and identified a drug that could normalize those responses.17 The DNB portfolio also includes research on non-pharmacological SUD treatments including transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and neurofeedback. Research on the interactions of complex neural circuits that underlie substance use, aversive responses to drugs that can inhibit drug-seeking, and interactions between neural and non-neuronal cells in these circuits is also supported in this portfolio. DNB funds technology development that enables studies of the functional organization of the living brain from cells to circuits to networks, and advanced computational approaches including theoretical modeling and novel methods for analyzing large, diverse data sets. One recent study found that activity in two different brain regions is linked with nicotine addiction severity and nicotine withdrawal, which is of particular interest because current smoking cessation treatments only affect one of those areas.18 Such studies can help inform the creation of new and improved treatments with basic data on neural circuits. Finally, DNB supports mechanistic research to address real-world challenges faced in clinical care of SUD, such as polysubstance use, co-occurring conditions, and sex and gender differences in the development of SUDs.
Intersection of COVID-19 and Substance Use Disorders
Significant increases in drug use and overdoses have been reported since March 2020, when the coronavirus disease 2019 (COVID-19) pandemic emerged and physical distancing policies were put into place. Recent findings show that people with SUD are more likely to develop COVID-19 and to experience worse outcomes than people without SUD. An analysis of a large nationwide sample of urine drug tests by Millennium Health showed steep increases following mid-March in the use of non-prescribed fentanyl (32 percent), methamphetamine (20 percent), heroin (13 percent), and cocaine (10 percent). Overdose reports increased an average of 18 percent following mid-March for 62 percent of counties in the Overdose Detection Mapping Application Program. Data from the CDC suggest an acceleration of overdose deaths during the pandemic, and the 12-months ending in May 2020 marked the highest number of overdose deaths ever recorded.
In March 2020, shortly after COVID-19 mitigation guidelines were released, NIDA issued a Notice of Special Interest for research on the intersection of COVID-19 and SUD. NIDA is supporting over 90 studies under this and other NIH COVID-19 funding opportunities, including basic research on severe acute respiratory syndrome coronavirus 2 (SARSCOV-2) infection, the effect of COVID-19 on people who use drugs, the effect of COVID-19 on child and adolescent development, and the impact of pandemic related policy changes on access to SUD treatment. The latter includes studies on how telemedicine affects OUD treatment and the impact of policies facilitating at-home methadone dosing on OUD treatment adherence. NIDA is supports four studies on COVID19 through its intramural program, including research to develop novel therapeutics for SARS-CoV-2 and assess the effects of pandemic-related psychosocial stress on patients in treatment for OUD.
NIDA leads an effort under the trans-NIH Rapid Acceleration of Diagnostics-Radical (RADx-Rad) initiative on methods to detect SARS-CoV-2 in wastewater to improve community-level surveillance of the virus. This project takes advantage of NIDA expertise on wastewater surveillance of drug use. NIDA also participates in the RADx-Underserved Populations (RADx-UP) initiative, which aims to expand COVID-19 testing among underserved populations, including those with SUD.
Continued investment in research on COVID-19 is vital for reducing devastating SUD and overdose outcomes, and NIDA continues to welcome applications to address the intersection of COVID-19 and SUD.
One of NIDA’s flagship basic science projects is the Adolescent Brain Cognitive Development (ABCD) study, which will follow children over 10 years, beginning at ages 9-10. Scientists are using techniques such as advanced brain imaging, interviews, and behavioral testing to determine how childhood experiences interact to affect brain development and—ultimately—social, behavioral, academic, and health outcomes, including substance use. Understanding how drugs interact with individual genetic, neurobiological, environmental, social, and developmental factors is essential to understanding what puts a person at risk for or confers resilience to addiction. Enrollment is complete with a total of 11,878 youth and their families participating. The study has already released baseline and one-year follow-up data from the full cohort, and more than 70 research papers have been published using these data, leading to a better understanding of the association between certain traits and experiences and brain structure and function, cognitive ability, and mental health. For example, a recent study has found that certain measures of obesity correlate with measurements of the density of an area of the brain responsible for motivation and reward, suggesting a possible neural mechanism for behavioral changes that lead to obesity.19
Budget Policy: The FY 2022 President’s Budget request is $603.8 million, an increase of $111.0 million or 22.5% percent compared with the FY 2021 Enacted level.
Epidemiology, Services, and Prevention Research
NIDA’s Division of Epidemiology, Services, and Prevention Research (DESPR) supports integrated approaches to understanding and addressing the interactions between individuals and environments that contribute to drug use, addiction, and related health problems. Through Monitoring the Future, the Population Assessment of Tobacco and Health study, and other studies, DESPR monitors trends in drug use, including marijuana, vaping/e-cigarettes, and other drugs, as well as the potential risks and health outcomes related to these behaviors.
Preventing the initiation of substance use to minimize risks of harmful consequences is an essential part of addressing SUD. To this end, DESPR funds a portfolio of prevention research to understand and intervene upon mechanisms that underlie risk for and resilience to addiction and common comorbidities. This includes studies on how biological, psychosocial, and environmental factors operate to enhance or mitigate an individual’s propensity to initiate substance use or to escalate from use to misuse to SUD across different developmental stages. This information, along with rapidly growing knowledge about substance use and addiction, is helping to inform the development of evidence-based prevention strategies.
DESPR also supports research on integrating prevention and treatment services into healthcare and community systems to reduce the burden of drug problems across the lifespan. For example, ongoing research is examining efforts to implement evidence-based SUD treatment in jails and prisons, expand the use of effective medications for OUD in primary care settings, develop strategies to reduce transmission of viral infections related to substance use (e.g., HIV, and Hepatitis C), and increase uptake and retention in treatment for SUD and HIV. DESPR also funds research into the efficacy of screening, brief intervention, and referral to treatment in primary care settings for reducing drug use and SUD.
Budget Policy: The FY 2022 President’s Budget request is $418.0 million, an increase of $76.9 million or 22.5 percent compared with the FY 2021 Enacted level.
Therapeutics and Medical Consequences Research
NIDA’s Division of Therapeutics and Medical Consequences (DTMC) supports research to evaluate the safety and efficacy of pharmacotherapies and devices to treat SUD. This work spans all phases of medical product development including synthesis and preclinical evaluation of potential therapeutics, clinical trial design and execution, and preparing regulatory submissions. Through these investments, NIDA helps to mitigate risks of developing new treatments for SUD. For example, in collaboration with US WorldMeds, DTMC supported clinical trials on LUCEMYRA™, the first medication targeted specifically to treat the physical symptoms associated with opioid withdrawal, which was approved by the FDA in May 2018. NIDA also supports research to identify promising compounds and make them more feasible for pharmaceutical companies to complete costly clinical studies for SUD indications. As part of the HEAL InitiativeSM, described below, DTMC leads efforts to develop new and repurposed medications to treat OUD.
NIDA is also prioritizing the development of pharmacological treatments for stimulant use disorders. This portfolio includes approaches from repurposing approved medications for other SUDs, to developing a novel monoclonal antibody that could prevent or reduce methamphetamine intoxication (see program portrait “Medications Development for Stimulant Use Disorder”).
Medications Development for Stimulant Use Disorder
Stimulants, including methamphetamine and cocaine, are highly addictive and associated with serious physical and mental health consequences. From 2012 - 2019, overdose deaths involving methamphetamine increased more than 6-fold, and overdose deaths involving cocaine more than tripled. The only currently available treatments for stimulant use disorders are behavioral therapies such as contingency management. Although cognitive behavioral therapy is often used, its effectiveness for treating methamphetamine use disorder has not been demonstrated. There are no U.S. Food and Drug Administration (FDA)-approved medications for stimulant use disorders or overdose.
NIDA’s portfolio in medications development for stimulant use disorders is multifaceted, spanning novel biological targets for new medications, to anti-cocaine and anti-meth vaccines, to the repurposing of existing medications. For example, NIDA-supported researchers are developing and testing novel compounds that target VMAT2, a protein that plays an important role in the transport and release of neurotransmitters including dopamine, which enables the reinforcing and addictive effects of stimulants. VMAT2 has been shown to decrease methamphetamine-seeking in animal models. Researchers are also working to develop vaccines that sequester stimulants in the blood so they do not reach the brain. As one example, a monoclonal antibody (IXT-m200, developed by InterveXion) for the treatment of methamphetamine use disorder and overdose is being studied in a Phase II clinical trial and received Fast Track designation from the FDA. Additional studies focus on long-acting enzymes that block the physiological and toxic effects of stimulants, such as cocaine hydrolase for cocaine use disorder. NIDA-supported researchers are also exploring medications approved for other indications to test their effectiveness in treating stimulant use disorders. For example, the recently completed Accelerated Development of Additive Pharmacology Treatment (ADAPT-2) trial demonstrated that bupropion plus naltrexone was effective for reducing methamphetamine use and craving in individuals with moderate to severe methamphetamine use disorder. In addition, numerous agonists and antagonists are being studied including mirtazapine, a drug approved for the treatment of depression, that has been shown to decrease methamphetamine use in a small randomized control trial.
Developing effective medications for stimulant use disorders is one of NIDA’s highest priorities and is critical to improving the treatment of people addicted to methamphetamine, cocaine, and other stimulants.
Budget Policy: The FY 2022 President’s Budget request is $142.3 million, an increase of $26.2 million or 22.5 percent compared with the FY 2021 Enacted level.
Clinical Trials Network Research
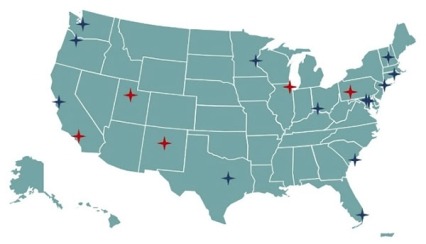
The overarching mission of the NIDA Clinical Trials Network (CTN) is to allow medical and specialty treatment providers, treatment researchers, patients, and NIDA to cooperatively develop, validate, refine, and deliver new treatment options to patients. The CTN comprises: 16 research nodes with 31 principal investigators affiliated with academic medical centers and large health care networks; two research coordinating centers; and more than 240 community-anchored treatment programs. This unique partnership enables the CTN to conduct studies of behavioral, pharmacological, and integrated treatment interventions in multisite clinical trials to determine effectiveness across a broad range of settings and populations. It also allows the CTN to ensure the transfer of research results to providers and patients. The network evaluates interventions, implementation strategies, and health system approaches to addressing SUD and co-occurring conditions such as mental illnesses and HIV. Using support from HEAL, the CTN has been able to expand its geographical reach (see Figure 2), adding 5 new nodes in 2020 that can develop and test interventions in new populations.
The CTN is conducting studies to evaluate strategies for integrating OUD screening and treatment into emergency departments, primary care clinics, and American Indian/Alaska Native communities. The CTN is also conducting a study to examine the effects of medications for OUD in pregnant women. It has supported studies to capture important data for research on SUD in electronic health record (EHR) systems in primary care and emergency departments, and is currently developing and testing a clinical decision support tool that integrates with EHR systems to help doctors diagnose OUD and provide treatment or refer patients to appropriate care. Complementing the work supported through NIDA’s DTMC, CTN studies are investigating the effectiveness and safety of pharmacotherapies (e.g., ADAPT-2; see program portrait, “Medications for Stimulant Use Disorder”), and transcranial magnetic stimulation for methamphetamine and cocaine use disorders.
Budget Policy: The FY 2022 President’s Budget request is $48.6 million, an increase of $8.9 million or 22.5 percent compared with the FY 2021 Enacted level.
Research Responding to the Opioid Crisis
Through the HEAL InitiativeSM, NIDA continues to expand its support for research to combat opioid addiction. For example, NIDA is supporting a study to prevent the high rate of opioid misuse initiation associated with the transition from adolescence to adulthood. HEAL funds are also being used to accelerate the availability of novel treatments for OUD and overdose, including to develop longer-acting formulations of existing OUD drugs like buprenorphine, repurpose approved drugs for other indications for OUD, and develop novel antibodies to prevent the action of opioids in the brain.
The HEAL InitiativeSM leveraged NIDA’s existing CTN to expand the network by adding 5 new nodes that are supporting the development of 26 new research protocols. Two large projects address knowledge gaps around treatment initiation and retention. The first is a study of the efficacy of prevention interventions to halt the progression from risky opioid use to OUD. Researchers will test the efficacy of a Subthreshold Opioid Use Disorder Prevention (STOP) intervention in primary care settings to identify and address early-stage opioid misuse. The second is a study to test strategies to improve retention in medication treatment for OUD, as well as strategies to improve outcomes for patients stabilized on OUD medications who want to stop taking them. This will be the first study of medications to treat OUD to follow prospectively a large sample of patients through discontinuation.
HEAL also supports studies that are developing effective implementation strategies for evidence-based interventions. The Justice Community Opioid Innovation Network (JCOIN) is testing strategies to expand effective OUD treatment and care for people in justice settings in partnership with local and state justice systems and community-based treatment providers, which will fully launch as clinical trials in early 2021. The HEALing Communities Study, a multisite implementation research study, is investigating coordinated approaches for deploying evidence-based strategies to prevent and treat opioid misuse and OUD tailored to the needs of local communities. The goal of the study is to reduce opioid-related overdose deaths by 40 percent over three years. Research sites are partnering with 67 communities highly affected by the opioid crisis in four states to measure the impact of these efforts.
Finally, the HEALthy Brain and Child Development Study is a NIDA and HEAL-led, trans-NIH effort to add to our understanding of early brain development trajectories. This study will establish a cohort of pregnant women and follow their children through the first decade of their lives to determine how environmental factors, including maternal drug exposure and genetics, influence early brain development and behavioral and clinical outcomes such as mental illnesses and addiction.
Budget Policy: The FY 2022 President’s Budget request for HEAL Initiative extramural research is $400.4 million, an increase of $134.5 above the FY 2021 Enacted level. Including $5.0 million for Research Management and Support, total NIDA funding in FY 2022 for the HEAL initiative is $405.4 million, an increase of $135.1 million or 50.0 percent compared to the FY 2021 Enacted level.
High-Tech Biomedical Product Development
NIDA’s Office of Translational Initiatives and Program Innovations (OTIPI) takes research discoveries in prevention, detection, and treatment of SUD into candidate health applications for commercialization. OTIPI manages NIDA’s Small Business Innovation Research/Small Business Technology Transfer Programs to advance health applications. It also uses novel fit-for-purpose funding authorities, such as Prizes and Open Competitions, and establishes teaching programs that equip scientists with the competence to translate advances in addiction research into products. Many of these efforts take the form of innovative new technology applications, from mobile apps that help patients find open beds in addiction treatment facilities or connect to support communities, to more sophisticated medical devices. (See the Program Portrait “NIDA’s Innovative Technology Portfolio.”)
NIDA’s Innovative Technology Portfolio
Twenty-first century problems require 21st century solutions, and addiction is no exception. New trends in technology, including mobile apps, GPS, portable biosensors, and virtual reality offer opportunities to innovate new approaches to both research and delivery of addiction prevention interventions and treatments. Unfortunately, the addiction space is relatively underserved when it comes to product development and commercialization. To address this critical gap, NIDA invests in a variety of translational initiatives, in order to help researchers commercialize their research or to encourage companies with promising technologies to expand to the addiction space.
Some of these products deliver evidence-based therapies in novel ways. For example, OpenBeds, a smartphone app originally designed to connect patients to open acute care beds, has been expanded with NIDA support to facilitate referrals to residential, inpatient, and outpatient addiction treatment facilities, and is currently being used by several state governments and hospital systems. One technology, a novel app developed by Sound Life Sciences, turns a user’s smartphone into a portable respiratory monitor capable of detecting changes in breathing associated with an overdose, sounding an alarm and alerting emergency services. Other apps help doctors and patients monitor and maintain their OUD medication, and connect individuals to behavioral therapies, peer support groups, and community interventions.
NIDA also supports the development of entirely novel technologies. One such technology is a hospital bassinet pad called Prapela SVS that applies gentle vibrations to soothe babies born dependent on opioids, which is currently seeking FDA approval. Another technology, applied VR, uses virtual reality as an alternative form of pain relief to opioids. Others help hospitals monitor their controlled substances for theft or diversion, identify illicit drug transactions on the internet, or find innovative new approaches to measuring pain via brain activity patterns.
Budget Policy: The FY 2022 President’s Budget request is $55.9 million, an increase of $10.3 million or 22.5 percent compared with the FY 2021 Enacted level.
Intramural Research Program
NIDA conducts research in high priority areas through its Intramural Research Program (IRP). The IRP portfolio includes research to: 1) elucidate the mechanisms underlying the development of SUDs; 2) evaluate potential new therapies for SUDs, including pharmacological and non-pharmacological interventions; and 3) identify and characterize emerging drugs such as synthetic opioids, stimulants, and cannabinoids.
One example of treatment evaluation at the IRP is a bench-to-bedside project in which IRP investigators are testing a novel compound to treat OUD that activates the same receptors as traditional opioids but has only a subset of their cellular actions. IRP investigators are testing whether the compound reduces self-administration of opioids in animal models and people with OUD, and whether it prevents opioid withdrawal with fewer side effects than medications in current use. If successful, this compound could be a new medication for OUD.
The IRP is also working with the National Center for Advancing Translational Sciences on a dopamine D3 receptor antagonist that could be taken together with opioid pain relievers to reduce the chance of developing OUD. Preliminary animal studies suggest that the compound reduces opioid self-administration and drug-seeking behavior without reducing the pain-relieving effects of opioids. This compound holds promise as an adjunct to opioid treatment for pain and potentially for OUD.
Non-pharmacological addiction treatments are also being developed in NIDA’s IRP. The on-site treatment-research clinic includes efforts to develop a smartphone app that uses machine learning to detect or predict stress, craving, and drug use within hours—and a parallel project to develop content that the app could deliver “just in time.” Because current apps purporting to serve these functions do not meet scientific standards of evidence, IRP is addressing a major gap in mobile health. Using passive measurement and digital phenotyping techniques, the IRP is also developing interventions and big data methodologies to prevent HIV transmission associated with unprotected sex in the context of substance use.
Budget Policy: The FY 2022 President’s Budget request is $105.2 million, an increase of $3.1 million or 3.0 percent compared with the FY 2021 Enacted level.
Research Management and Support
Research Management and Support activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. RMS staff at NIDA play leadership roles in helping to coordinate NIDA’s involvement in the NIH HEAL InitiativeSM, spearheading NIH’s response to the opioid overdose epidemic.
In addition to the infrastructure required to support research and training, NIDA strives to provide evidence-based resources and educational materials about substance use and addiction, including information about timely public health topics such as opioid overdose prevention, marijuana research, use and consequences of vaping, synthetic drug trends, and medications for treatment of SUD, including OUD. To this end, the RMS portfolio incorporates education and outreach activities to inform public health policy and practice with the goal of ensuring that NIDA is the primary trusted source for scientific information on drug use and addiction. Staff supported by NIDA’s RMS budget coordinate key activities that help to train the next generation of addiction scientists. In addition, NIDA’s RMS portfolio includes the NIDAMED initiative,21 which is aimed at engaging and educating clinicians in training and in practice in the latest science related to drug use and addiction.
Budget Policy: The FY 2022 President’s Budget request is $78.3 million, an increase of $1.3 million or 1.7 percent compared with the FY 2021 Enacted level.
Medications Development for Stimulant Use Disorder
Stimulants, including methamphetamine and cocaine, are highly addictive and associated with serious physical and mental health consequences. From 2012 - 2019, overdose deaths involving methamphetamine increased more than 6-fold, and overdose deaths involving cocaine more than tripled. The only currently available treatments for stimulant use disorders are behavioral therapies such as contingency management. Although cognitive behavioral therapy is often used, its effectiveness for treating methamphetamine use disorder has not been demonstrated. There are no U.S. Food and Drug Administration (FDA)-approved medications for stimulant use disorders or overdose.
NIDA’s portfolio in medications development for stimulant use disorders is multifaceted, spanning novel biological targets for new medications, to anti-cocaine and anti-meth vaccines, to the repurposing of existing medications. For example, NIDA-supported researchers are developing and testing novel compounds that target VMAT2, a protein that plays an important role in the transport and release of neurotransmitters including dopamine, which enables the reinforcing and addictive effects of stimulants. VMAT2 has been shown to decrease methamphetamine-seeking in animal models. Researchers are also working to develop vaccines that sequester stimulants in the blood so they do not reach the brain. As one example, a monoclonal antibody (IXT-m200, developed by InterveXion) for the treatment of methamphetamine use disorder and overdose is being studied in a Phase II clinical trial and received Fast Track designation from the FDA. Additional studies focus on long-acting enzymes that block the physiological and toxic effects of stimulants, such as cocaine hydrolase for cocaine use disorder. NIDA-supported researchers are also exploring medications approved for other indications to test their effectiveness in treating stimulant use disorders. For example, the recently completed Accelerated Development of Additive Pharmacology Treatment (ADAPT-2) trial demonstrated that bupropion plus naltrexone was effective for reducing methamphetamine use and craving in individuals with moderate to severe methamphetamine use disorder. In addition, numerous agonists and antagonists are being studied including mirtazapine, a drug approved for the treatment of depression, that has been shown to decrease methamphetamine use in a small randomized control trial.
Developing effective medications for stimulant use disorders is one of NIDA’s highest priorities and is critical to improving the treatment of people addicted to methamphetamine, cocaine, and other stimulants.
NIDA’s Innovative Technology Portfolio
Twenty-first century problems require 21st century solutions, and addiction is no exception. New trends in technology, including mobile apps, GPS, portable biosensors, and virtual reality offer opportunities to innovate new approaches to both research and delivery of addiction prevention interventions and treatments. Unfortunately, the addiction space is relatively underserved when it comes to product development and commercialization. To address this critical gap, NIDA invests in a variety of translational initiatives, in order to help researchers commercialize their research or to encourage companies with promising technologies to expand to the addiction space.
Some of these products deliver evidence-based therapies in novel ways. For example, OpenBeds, a smartphone app originally designed to connect patients to open acute care beds, has been expanded with NIDA support to facilitate referrals to residential, inpatient, and outpatient addiction treatment facilities, and is currently being used by several state governments and hospital systems. One technology, a novel app developed by Sound Life Sciences, turns a user’s smartphone into a portable respiratory monitor capable of detecting changes in breathing associated with an overdose, sounding an alarm and alerting emergency services. Other apps help doctors and patients monitor and maintain their OUD medication, and connect individuals to behavioral therapies, peer support groups, and community interventions.
NIDA also supports the development of entirely novel technologies. One such technology is a hospital bassinet pad called Prapela SVS that applies gentle vibrations to soothe babies born dependent on opioids, which is currently seeking FDA approval. Another technology, applied VR, uses virtual reality as an alternative form of pain relief to opioids. Others help hospitals monitor their controlled substances for theft or diversion, identify illicit drug transactions on the internet, or find innovative new approaches to measuring pain via brain activity patterns.
References
- www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- https://emergency.cdc.gov/han/2020/han00438.asp
- https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- pubmed.ncbi.nlm.nih.gov/32929211/
- Teens using vaping devices in record numbers
- Vaping marijuana use in 2019 rose in college age adults
- pubmed.ncbi.nlm.nih.gov/31783934/
- pubmed.ncbi.nlm.nih.gov/31688891/
- www.fda.gov/media/133880/download
- Access to addiction services differs by race and gender
- www.ncbi.nlm.nih.gov/pmc/articles/PMC3670657/, pubmed.ncbi.nlm.nih.gov/16327107/, www.ncbi.nlm.nih.gov/pmc/articles/PMC3665009/
- heal.nih.gov/research/research-to-practice/jcoin
- heal.nih.gov/research/infants-and-children/healthy-brain
- projectreporter.nih.gov/project_info_description.cfm?aid=9969974&icde=51760156
- projectreporter.nih.gov/project_info_details.cfm?aid=10022112&icde=51760006
- pubmed.ncbi.nlm.nih.gov/31611707/
- europepmc.org/article/med/22493758
- pubmed.ncbi.nlm.nih.gov/31816020/
- NIDA supported science leads to first FDA approved medication for opioid withdrawal
- NIDAMED