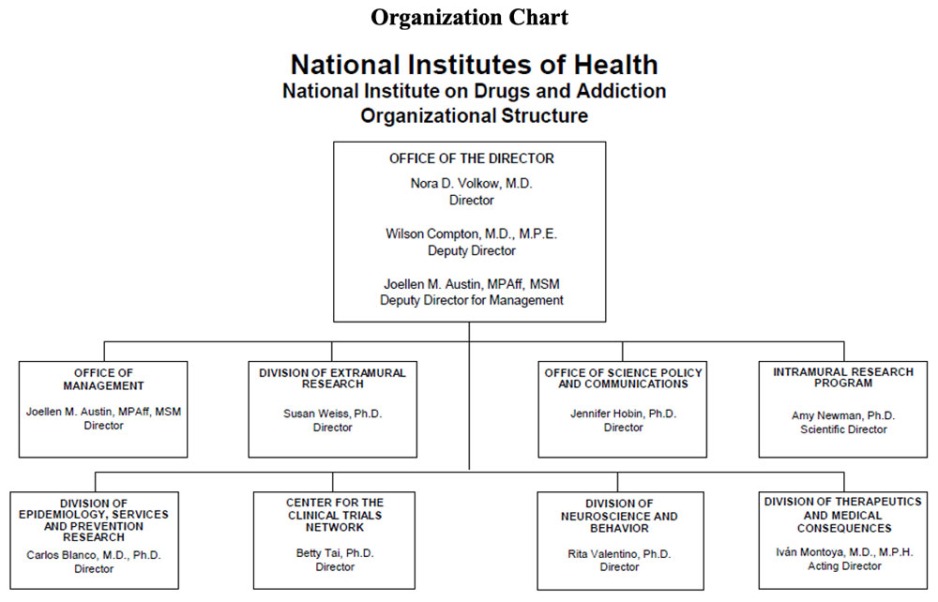
Organizational Chart
- Text Description of Organizational Chart
Office of the Director
- Nora D. Volkow, M.D.
Director - Wilson Compton, M.D., M.P.E.
Deputy Director - Joellen M. Austin, MPAff, MSM
Deputy Director for Management
Office of Management
- Joellen M. Austin, MPAff, MSM
Director
Division of Extramural Research
- Susan Weiss, Ph.D.
Director
Office of Science Policy and Communications
- Jennifer Hobin, Ph.D.
Director
Intramural Research Program
- Amy Newman, Ph.D.
Scientific Director
Division of Epidemiology, Services and Prevention Research
- Carlos Blanco, M.D., Ph.D.
Director
Center for the Clinical Trials Network
- Betty Tai, Ph.D.
Director
Division of Neuroscience and Behavior
- Rita Valentino, Ph.D.
Director
Division of Therapeutics and Medical Consequences
- Ivan Montoya, M.D., M.P.H.
Acting Director
- Nora D. Volkow, M.D.
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to drugs and addiction, $1,843,326,000.
Tables
- Budget Mechanism
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2021
FinalFY 2022 CR FY 2023
President's
BudgetFY 2023
+/-
FY 2022
CRNo. Amount No. Amount No. Amount No. Amount Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Research Projects: Noncompeting 950 $678,577 951 $700,315 678 $607,669 -273 -$92,645 Administrative Supplements (180) 19,448 (133) 11,000 (166) 13,000 (33) 2,000 Competing: Renewal 18 16,607 20 18,000 27 25,000 7 7,000 New 291 191,675 263 187,423 782 587,324 519 399,901 Supplements 0 0 0 0 0 0 0 0 Subtotal, Competing 309 $208,282 283 $205,423 809 $612,324 526 $406,901 Subtotal, RPGs 1,259 $906,307 1,234 $916,737 1,487 $1,232,993 253 $316,256 SBIR/STTR 70 41,373 80 46,727 96 57,073 16 10,346 Research Project Grants 1,329 $947,681 1,314 $963,465 1,583 $1,290.066 269 $326,602 Research Centers: Specialized / Comprehensive 27 $58,229 25 $53,562 33 $70,444 8 $16,882 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 200 0 0 0 0 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 27 $58,429 25 $53,562 33 $70,444 8 $16,882 Other Research: Research Careers 236 $44,880 236 $44,822 236 $44,786 0 -$36 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 27 97,628 23 74,089 44 74,744 21 654 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 1,297 0 1,900 0 1,900 0 0 Other 68 27,191 67 26,824 75 29,438 8 2,614 Other Research 331 $170,996 326 $147,636 355 $150,867 29 $3,232 Total Research Grants 1,687 $1,177,106 1,665 $1,164,662 1,971 $1,511,378 306 $346,716 Individual Awards 149 $6,741 167 $7,656 166 $7,802 -1 $145 Institutional Awards 367 20,585 352 20,051 374 20,432 22 381 Total Research Training 516 $27,326 519 $22,707 540 $28,233 21 $526 Research and Development Contracts 88 $93,763 85 $102,600 87 $114,615 2 $12,015 (SBIR/STTR) (non-add) (4) (6,584) (0) (495) (0) (3,602) (0) (3,107) -Intramural Research 121 101,528 123 104,191 123 106,800 0 2,609 Res. Management & Support 268 76,144 275 80,500 275 82,300 0 1,800 Res. Management & Support (SBIR Admin) (non-add) (0) (235) (0) (300) (0) (300) (0) (0) Construction 0 0 0 0 0 0 0 0 Buildings and Facilities 0 0 0 0 0 0 0 0 Total, NIDA 389 $1,475,867 398 $1,479,660 398 $1,843,326 0 $363,666 1 All numbers in italics and brackets are non-add entries.
- Amounts Available for Obligation
(Dollars in Thousands)1 Source of Funding FY 2021
FinalFY 2022
CRFY 2023
President's
BudgetAppropriation $1,479,660 $1,479,660 $1,843,326 Secretary's Transfer -$4,442 $0 $0 OAR HIV/AIDS Transfers $649 $0 $0 Subtotal, adjusted budget authority $1,475,867 $1,479,660 $1,843,326 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $1,475,867 $1,479,660 $1,843,326 Unobligated balance lapsing -62 0 0 Total obligations $1,475,805 $1,479,660 $1,843,326 1Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2021 - $83,548 FY 2022 - $92,005 FY 2023 - $76,448- Summary of Changes
(Dollars in Thousands) FY 2022 CR $1,479,660 FY 2023 President's Budget $1,843,326 Net change $363,666 FY 2022 CR FY 2023 President's Budget Built-In Change from
FY 2022 CRCHANGES FTEs/Budget Authority FTEs/Budget Authority FTEs/Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of January 2022 pay increase & benefits $32,151 $33,332 $214 b. January FY 2023 pay increase & benefits 32,151 33,332 1,091 c. Paid days adjustment 32,151 33,332 -122 d. Differences attributable to change in FTE 32,151 33,332 266 e. Payment for centrally furnished services 12,547 12,798 251 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs$59,493 $60,670 $1,241 Subtotal $2,939 2. Research Management and Support: a. Annualization of January 2022 pay
increase & benefits$45,451 $47,065 $301 b. January FY 2023 pay increase & benefits 45,451 47,065 1,537 c. Paid days adjustment 45,451 47,065 -173 d. Differences attributable to change in FTE 45,451 47,065 0 e. Payment for centrally furnished services 5,740 5,855 115 f. Cost of laboratory supplies,
materials, other expenses, and
non-recurring costs$29,309 $29,381 $606 Subtotal $2,386 Subtotal, Built-in $5,325 Summary of Changes - Continued
(Dollars in Thousands)FY 2022 CR FY 2023 President's Budget Program Change from
FY 2022 CRCHANGES No. Amount No. Amount No. Amount B. Program: 1. Research Project Grants: a. Noncompeting 951 $711,315 678 $620,669 -273 -$90,645 b. Competing 283 $205,423 809 $612,324 526 $406,901 c. SBIR/STTR 80 $46,727 96 $57,073 16 $10,346 Subtotal, RPGs 1,314 $963,465 1,583 $1,290,066 269 $326,602 2. Research Centers 25 $53,562 33 $70,444 8 $16,882 3. Other Research 326 $147,636 355 $150,867 29 $3,232 4. Research Training 519 $27,707 540 $28,233 21 $526 5. Research and development contracts 85 $102,600 87 $114,615 2 $12,015 Subtotal, Extramural $1,294,969 $1,654,226 $359,257 FTEs FTEs FTEs 6. Intramural Research 123 $104,191 123 $106,800 0 -$330 7. Research Management and Support 275 $80,500 275 $82,300 0 -$586 8. Construction 0 0 0 9. Buildings and Facilities 0 0 0 Subtotal, program 398 $1,479,660 398 $1,843,326 0 $358,341 Total built-in and program changes $363,666 - Authorizing Legislation
PHS Act/Other Citation U.S. Code Citation 2022 Amount Author-
izedFY 2022 CR 2023 Amount Author
-izedFY 2023 President's Budget Research and Investigation Section 301 42§241 Indefinite $1,479,660,000 Indefinite $1,843,326,000 National Institute on Drugs and Addiction Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,479,660,000 $1,843,326,000 - Appropriations History
Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2014 $1,071,612,000 $1,064,490,000 $1,025,435,000 Rescission $0 2015 $1,023,268,000 $1,028,614,000 Rescission $0 2016 $1,047,397,000 $1,050,875,000 $1,069,086,000 $1,077,488,000 Rescission $0 20171 $1,050,550,000 $1,107,700,000 $1,103,032,000 1,090,853,000 Rescission $0 2018 $864,998,000 $1,107,497,000 $1,113,442,000 $1,383,603,000 Rescission $0 2019 $1,137,403,000 $1,400,126,000 $1,420,591,000 $1,419,844,000 Rescission $0 2020 $1,296,379,000 $1,489,237,000 $1,490,498,000 $1,462,016,000 Rescission $0 2021 $1,431,770,000 $1,476,590,00 $1,505,192,000 $1,479,660,000 Rescission $0 2022 $1,852,503,000 $1,860,329,000 $1,832,906,000 $1,479,660,000 Rescission $0 2023 $1,843,326,000 1Budget Estimate to Congress includes mandatory financing.
- Budget Authority by Activity
(Dollars in thousands)1 Extramural Research FY 2021
FinalFY 2022 CR FY 2023
President's
BudgetFY 2023
+/-
FY 2022 CRFTE Amount FTE Amount FTE Amount FTE Amount Detail: Division of Therapeutics and Medical Consequences $111,777 $111,738 $135,976 $24,238 Division of Neuroscience and Behavior 496,067 495,894 603,460 107,566 Division of Epidemiology, Services and Prevention Research 346,342 346,222 421,322 75,100 Center for the Clinical Trials Network 39,461 39,447 48,004 8,557 Office of Translational Initiatives and Program Innovations 39,886 39,873 48,522 8,649 HEAL Initiative2 264,662 261,795 396,943 135,148 Subtotal, Extramural $1,298,195 $1,294,969 $1,654,226 $359,257 Intramural Research 121 $101,528 123 $104,191 123 $106,800 0 $2,609 Research Management & Support 268 $76,144 275 $80,500 275 $82,300 0 $1,800 TOTAL 389 $1,475.867 398 $1,479,660 398 $1,843,326 0 $363,666 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Total for HEAL Initiative including RMS is (in thousands) $269,484 in FY 2021, $270,295 in FY 2022, and $405,443 in FY 2023.- Budget Authority by Object Class
(Dollars in Thousands)1 FY 2022 CR FY 2023 President's Budget FY 2023 +/- FY 2022 OBJECT CLASSES FY 2022 CR FY 2023 President's Budget FY 2023 +/- FY 2022 Total compensable workyears: Full-time equivalent 398 398 0 Full-time equivalent of overtime and holiday hours 0 0 0 Average ES salary $206 $213 $7 Average GM/GS grade 13.0 13.0 0.0 Average GM/GS salary $130 $134 $5 Average salary, Commissioned Corps (42 U.S.C. 207) $112 $117 $5 Average salary of ungraded positions $150 $157 $6 Personnel Compensation: 11.1 Full-time permanent $34,521 $35,763 $1,242 11.3 Other than full-time permanent 14,761 15,313 552 11.5 Other personnel compensation 2,106 2,185 79 11.7 Military personnel 620 643 23 11.8 Special personnel services payments 6,548 6,793 245 11.9 Subtotal Personnel Compensation $58,556 $60,697 $2,141 12.1 Civilian personnel benefits 18,619 19,256 637 12.2 Military personnel benefits 427 443 16 13.0 Benefits to former personnel 0 0 0 Subtotal Pay Costs $77,602 $80,397 $2,795 21.0 Travel and transportation of persons 250 705 455 22.0 Transportation of things 88 90 2 23.1 Rental payments to GSA 0 0 0 23.2 Rental payments to others 0 0 0 23.3 Communications, utilities and misc. charges 144 147 3 24.0 Printing and reproduction $1 0 -$1 25.1 Consulting services 74,696 65,014 -9,681 25.2 Other services 18,151 17,259 -892 25.3 Purchase of goods and services from government accounts 103,791 117,674 13,882 25.4 Operation and maintenance of facilities 233 233 0 25.5 Research and development contracts 4,653 8,896 4,242 25.6 Medical care 350 365 14 25.7 Operation and maintenance of equipment 3,789 3,873 83 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal Other Contractual Services $205,664 $213,313 $7,649 26.0 Supplies and materials 4,559 4,659 100 31.0 Equipment 4,267 4,359 92 32.0 Land and structures 0 0 0 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions 1,187,085 1,539,655 352,570 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends 2 2 0 44.0 Refunds 0 0 0 Subtotal Non-Pay Costs $1,402,058 $1,762,929 $360,871 Total Budget Authority by Object Class $1,479,660 $1,843,326 $363,666 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses
(Dollars in Thousands) Object Classes FY 2022 CR FY 2023 President's Budget FY 2023 +/- FY 2022 Personnel Compensation: Full-time permanent (11.1) $34,521 $35,763 $1,242 Other than full-time permanent (11.3) 14,761 15,313 552 Other personnel compensation (11.5) 2,106 2,185 79 Military personnel (11.7) 620 643 23 Special personnel services payments (11.8) 6,548 6,793 245 Subtotal Personnel Compensation (11.9) $58,556 $60,697 $2,141 Civilian personnel benefits (12.1) $18,619 $19,256 $637 Military personnel benefits (12.2) 427 443 16 Benefits to former personnel (13.0) 0 0 0 Subtotal Pay Costs $77,602 $80,397 $2,795 Travel and transportation of persons (21.0) $250 $705 $455 Transportation of things (22.0) 88 90 2 Rental payments to others (23.2) 0 0 0 Communications, utilities and miscellaneous charges (23.3) 144 147 3 Printing and reproduction (24.0) 1 0 -1 Other Contractual Services: Consultant services (25.1) 26,534 25,752 -781 Other services (25.2) 18,151 17,259 -892 Purchases of goods and services from government accounts (25.3) 54,051 58,561 4,510 Operation and maintenance of facilities (25.4) 233 233 0 Operation and maintenance of equipment (25.7) 3,789 3,873 83 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $102,758 $105,678 $2,920 Supplies and materials (26.0) $4,559 $4,659 $100 Subtotal Non-Pay Costs $107,799 $111,279 $3,480 Total Administrative Costs $185,401 $191,676 $6,275 - Details of Full-Time Equivalent Employment (FTEs)
Office/
DivisionFY 2021
FinalFY 2022 CR FY 2023
President's BudgetCivilian Military Total Civilian Military Total Civilian Military Total Office of the Director Direct: 25 0 25 27 0 27 27 0 27 Total: 25 0 25 27 0 27 27 0 27 Division of Extramural Research Direct: 44 0 44 42 0 42 42 0 42 Total: 44 0 44 42 0 42 42 0 42 Office of Management Direct: 29 0 29 29 0 29 29 0 29 Reimbursable: 51 0 51 56 0 56 56 0 56 Total: 80 0 80 85 0 85 85 0 85 Office of Science Policy and Communication Direct: 23 0 23 25 0 25 25 0 25 Total: 23 0 23 25 0 25 25 0 25 Division of Epidemiology, Services and Prevention Research Direct: 27 1 28 28 1 29 28 1 29 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 27 1 28 28 1 29 28 1 29 Division of Neuroscience and Behavior Direct: 25 0 25 25 0 25 25 0 25 Total: 25 0 25 25 0 25 25 0 25 Division of Therapeutics and Medical Consequences Direct: 29 0 29 28 0 28 28 0 28 Total: 29 0 29 28 0 28 28 0 28 Center for the Clinical Trials Network Direct: 14 0 14 14 0 14 14 0 14 Total: 14 0 14 14 0 14 14 0 14 Intramural Research Program Direct: 116 4 120 118 3 121 120 2 122 Reimbursable: 1 0 1 2 0 2 1 0 1 Total: 117 4 121 120 3 123 121 2 123 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 384 5 389 394 4 398 395 3 398 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2019 13.3 2020 13.1 2021 13.0 2022 13.0 2023 13.0 - Detail of Positions
Detail of Positions1 GRADE FY 2021 Final FY 2022 CR FY 2023 President's Budget Total, ES Positions 1 1 1 Total, ES Salary $199,300 $206,275 $213,495 GM/GS-15 61 63 63 GM/GS-14 72 74 76 GM/GS-13 93 97 96 GS-12 41 36 38 GS-11 20 21 21 GS-10 0 0 0 GS-9 7 6 5 GS-8 8 8 8 GS-7 7 7 7 GS-6 1 1 1 GS-5 1 1 0 GS-4 0 0 0 GS-3 0 0 0 GS-2 0 0 0 GS-1 0 0 0 Subtotal 311 314 315 Commissioned Corps (42 U.S.C. 207): Assistant Surgeon General 0 0 0 Director Grade 2 1 1 Senior Grade 2 2 2 Full Grade 1 1 0 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 5 4 3 Ungraded 93 112 112 Total permanent positions 314 318 318 Total positions, end of year 410 431 431 Total full-time equivalent (FTE) employment, end of year 389 398 398 Average ES salary $199,300 $206,275 $213,495 Average GM/GS grade 13.0 13.0 13.0 Average GM/GS salary $125,382 $129,770 $134,312 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
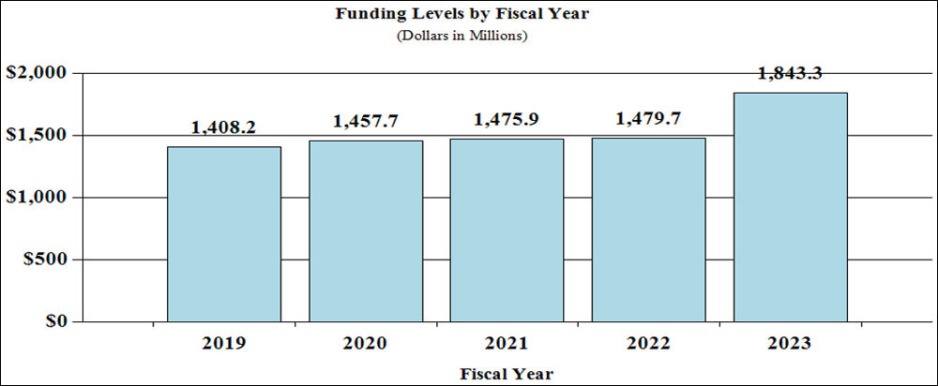
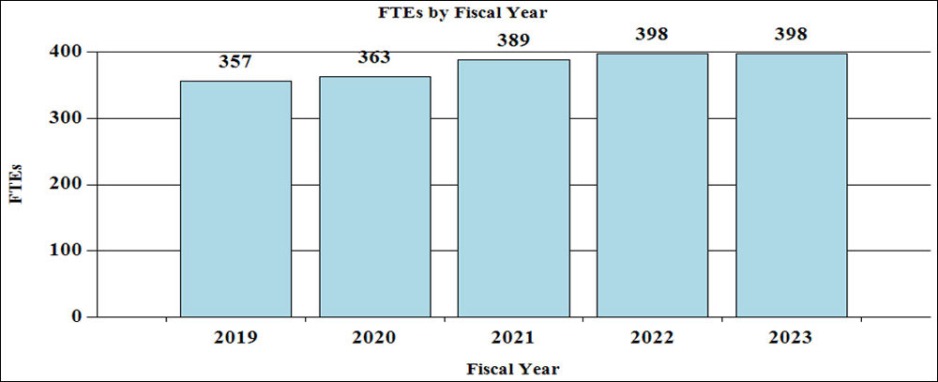
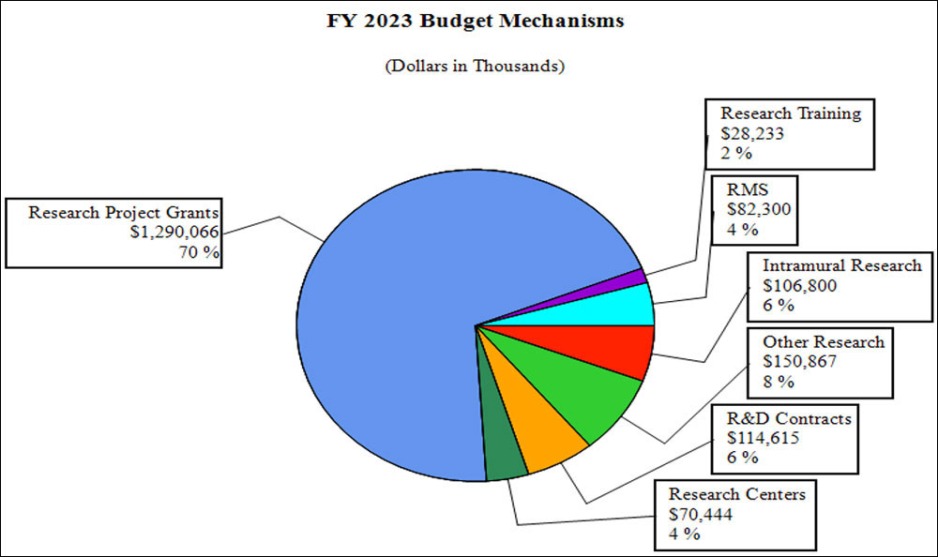
Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism (dollars in thousands):
Change by Selected Mechanism:
Justification of Budget Request
National Institute on Drugs and Addiction
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2021 Final | FY 2022 CR | FY 2023 President's Budget | FY 2023 +/- FY 2022 | |
|---|---|---|---|---|
| BA | $1,457,867,000 | $1,479,660,000 | $1,843,326,000 | +363,666,000 |
| FTE | 389 | 398 | 398 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2023 President’s Budget request is $1,843.3 million, an increase of $363.7 million or 24.6 percent compared with the FY 2022 Continuing Resolution (CR) level. Within this funding level, funding for the HEAL Initiative® will increase by $135.1 million or 50.0 percent compared with the FY 2022 CR level. In addition, funding for research into opioids and pain management outside the HEAL Initiative® will increase by an additional $196.3 million.
Major Changes in the Fiscal Year 2023 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2023 President’s Budget. The FY 2023 President’s Budget for NIDA is $1,843.3 million, an increase of $363.6 million above the FY 2022 CR level.
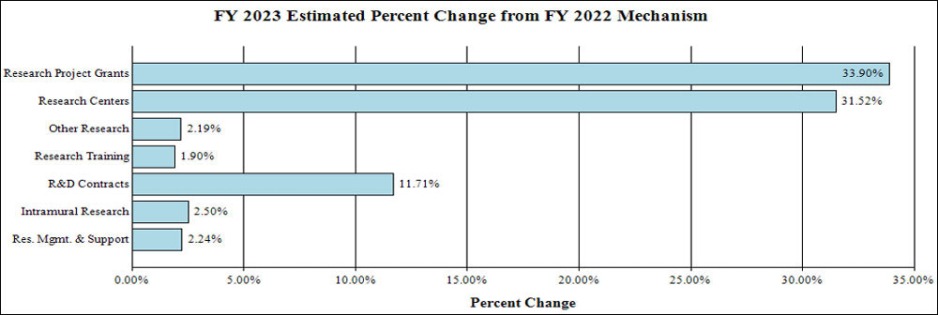
- Research Project Grants (RPGs) (+$326.6 million; total $1,290.1 million): NIDA will significantly increase funding for competing RPGs in support of the additional funding requested for the HEAL Initiative and related research into opioids and pain management. The number of noncompeting RPGs will decrease by 273 in FY 2023 as previously awarded projects are completed but the number of competing RPGs is expected to increase by over 500 in comparison to the FY 2022 level of 283 awards. The amount of support to competing awards will be increased by $406.9 million from FY 2022, a 198 percent increase.
- Research Centers (+$16.9 million; total $70.4 million): NIDA will increase support to new specialized/comprehensive centers projects.
- Other Research (+$3.2 million; total $150.9 million): NIDA will increase support to research career development awards, research training awards, and cooperative clinical research.
- Ruth L Kirchstein Training (+$526,430; total $28.2 million): NIDA will increase its numbers of full time training positions to 540, an increase of 21 positions in comparison to the FY 2022 CR level.
Director's Overview

NIDA Director
The National Institute on Drugs and Addiction (NIDA)2 is the lead federal agency supporting scientific research on drug use and its consequences. Its mission is to advance science on drug use and addiction and apply that knowledge to improve individual and public health. NIDA-supported research has led to the development of effective interventions for substance use and use disorders, but new and improved approaches and implementation strategies will be essential to combatting the addiction public health crisis. While decades of research have demonstrated that addiction is a chronic, treatable brain disorder influenced by environmental conditions, pervasive stigma against people with substance use disorders (SUDs) remains a major barrier to treatment. Language is a powerful driver of negative bias; even the word “abuse,” may perpetuate stigma against people who use drugs and deter people with SUD from seeking treatment. As a step toward reducing that stigma, NIDA is proposing that the Institute be renamed the “National Institute on Drugs and Addiction.”
Collision of Public Health Crises
The addiction and overdose crisis has collided with the COVID-19 pandemic. Since early in the pandemic, studies have found increases in the use of many kinds of drugs, including fentanyl, cocaine, heroin, methamphetamine, cannabis, and alcohol. Findings from the NIDA-supported Monitoring the Future (MTF) survey offer a window into how drug use among young people changed during the pandemic. In 2020, the use of amphetamines, inhalants, and over-the-counter cough medicine containing the opioid codeine increased among eighth graders.3 Marijuana use did not significantly change among adolescents, but increased to an all-time high among college-age adults, with 44 percent reporting marijuana use in the past year. Past year use of hallucinogens among college students also increased, reaching the highest level since 1982.4
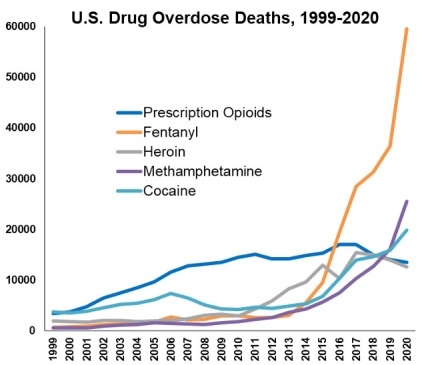
Especially alarming has been the increase in drug overdose deaths. Data from the Centers for Disease Control and Prevention (CDC) show that a record high of nearly 92,000 people died of overdose in the United States in 2020, an unprecedented one-year increase of 30 percent. Overdose deaths continue to be dominated by opioids, mainly fentanyl and fentanyl analogs, and stimulants continue to be an increasing overdose threat (see Figure 1). Overdose death rates in the last year increased by 47 percent for the methamphetamine category, and over 23 percent for cocaine.5 Social isolation and stress – factors long known to drive substance use and relapse – may be contributing to these increases. In addition, people who use drugs are more vulnerable to SARS-CoV-2 infection and to worse outcomes after infection. This is especially true for people with opioid use disorder (OUD) and Black individuals.6 The urgency of the overdose crisis requires that we continue our investments in prevention and treatment while addressing challenges such as COVID-19 and racial health disparities. In parallel, we must maintain support for basic research to inform intervention development and research to effectively implement evidence-based interventions in diverse settings.
Responding to the COVID-19 Pandemic
NIDA is supporting over 100 studies at the intersection of COVID-19 and substance use, including research on the impact of COVID-19 on brain and child development; the impact of COVID-19-related policy changes such as expanded access to telemedicine and take-home doses of methadone, treatment access and outcomes; comorbid physical and psychiatric conditions; vaccine hesitancy; and basic research on COVID-19 infection. NIDA’s National Drug Early Warning System (NDEWS) expanded in 2020 to 18 sentinel sites, incorporating real-time surveillance of drugs and data on substance use-related consequences of COVID-19. NIDA also leads a trans-NIH initiative for early detection of SARS-CoV-2 in wastewater and participates in other NIH-wide COVID-19 initiatives examining ways to expand testing and understand the impact of COVID on high-risk and underserved populations, including people who use drugs.
Addressing Racial Health Disparities
The COVID-19 pandemic highlighted racial health disparities that are particularly stark in the field of addiction, where punitive approaches to drug use have disproportionately affected Black individuals and other communities of color.7 Moreover, the fastest increases in opioid overdose deaths are among Black Americans,8,9 and children of minority groups are more likely to have lost a parent to COVID-19 than white children.10 To address these disparities, in July 2020 NIDA established its Racial Equity Initiative to eliminate racism in NIDA’s workplace, scientific workforce, and research portfolio. NIDA is tracking its minority health and health disparities portfolios to identify gaps and create targeted funding opportunities to which $100 million will be dedicated over 10 years. NIDA is funding research on the impact of racism on drug use outcomes, ameliorating disparities in SUD care, and implementing culturally-tailored interventions. In addition, researchers are developing solutions to address digital inequalities in communities heavily impacted by drug addiction. NIDA is also increasing its support for highly meritorious projects carried out by scientists from underrepresented minority groups.
Leveraging Foundational Research to Inform Interventions
NIDA supports two large, longitudinal studies that leverage sophisticated brain imaging technologies and big data analytics to predict vulnerabilities to substance use and SUD, and facilitate personalized prevention interventions. The Healthy Brain and Child Development (HBCD) Study and Adolescent Brain Cognitive Development (ABCD) Study seek to uncover how biological, psychosocial, and environmental factors during development enhance or mitigate an individual’s propensity to initiate substance use or develop an SUD. Under the NIH Helping to End Addiction Long-Term (HEAL) Initiative®, the HBCD Study, launched in 2021, is following approximately 7,500 children from the prenatal period through ages 9-10 and includes research on the effects of COVID-19 during pregnancy on fetal brain development and other outcomes. The ABCD Study, which launched in 2015, is the largest long-term study of brain development and child health ever conducted, following nearly 12,000 children ages 9-10 through adolescence into young adulthood. The 2020 ABCD data release included survey data on the impact of COVID-19 and racism. Nearly 200 papers have already been published from this project, with topics covering genetic risk for SUDs, effects of adverse social environments including discrimination, poverty, and neighborhood deprivation on educational and brain development, early identification of risk for psychiatric conditions and symptoms, effects of screen time, and the interactions of these factors. The ABCD study is also investigating how pandemic-related social circumstances affect education and mental health for adolescents.
NIDA’s prevention portfolio builds on knowledge about risk and protective factors derived from studies like HBCD, ABCD, and others, to inform prevention strategies for substance use, use disorder, and related outcomes in diverse systems and for understudied populations. Prevention research funded by NIDA tests interventions in settings where at-risk individuals can be reached, such as health care, justice, school, and child welfare systems. NIDA-led prevention research supported by the HEAL Initiative® is testing effective interventions to prevent opioid misuse and OUD among adolescents and young adults, with a focus on vulnerable populations such as American Indian/Alaska Native (AI/AN) and Black communities. One example is a video game intervention to improve attitudes and knowledge related to risk behaviors among adolescents.
Developing Novel Therapeutics
NIDA-supported research has led to effective medications for OUD and opioid overdose. As these medications are underutilized, and as there are no U.S. Food and Drug Administration (FDA)-approved medications to treat stimulant use disorders, NIDA continues to support research on medication treatments. Extended-release methadone and new formulations of buprenorphine are being tested to improve patient retention in treatment. Research into non-opioid medications for OUD includes compounds that target dopamine D3 receptors expressed at greater levels in people with addiction, and kappa opioid receptors expressed in brain areas controlling reward. Other compounds target vesicular monoamine transporter 2, a protein that enables the addictive effects of stimulants. Additional research includes vaccines and monoclonal antibodies to combat OUD, stimulant use disorders, and overdose. This includes the first-ever Phase I clinical trial of a vaccine against opioids, and a clinical trial for a monoclonal antibody against methamphetamine. (See Program Portrait “Immunotherapies for Substance Use Disorders and Overdose.”) Recent research showed that using two existing medications, buproprion with naltrexone, reduced drug use in people with methamphetamine use disorder. NIDA funds research to address fentanyl overdose, such as higher dose naloxone and longer acting opioid antagonists.
NIDA invests in technologies such as neuromodulation to target brain circuits altered by drugs, and smartphone apps that detect overdose and contact emergency services, deliver cognitive therapy, connect people with care, and enable remote methadone prescribing and COVID-19 screening. Other technologies include a hospital bassinet that delivers soothing vibrations to infants born dependent on opioids, a virtual reality alternative to preoperative pain relief, and a system to monitor controlled substances in hospitals. A device to sense opioid overdose, automatically inject naloxone, and alert first responders is being tested, as well as data science approaches to analyze electronic health records to inform personalized treatment. NIDA also funds research to speed progress toward the development of novel targets for SUD medications.
Novel Approaches to Address the Complexities of Addiction
Chronic pain and OUD are commonly comorbid, but there is a lack of integrated treatments for these conditions, particularly for marginalized individuals in under-resourced settings. Through the HEAL Initiative®, NIDA is establishing the Integrative Management of chronic Pain and OUD for Whole Recovery (IMPOWR) Network. Clinical research centers across the country will develop effective interventions, best models of care for service delivery, and sustainable implementation strategies for access to quality care for patients, with an emphasis on diverse, vulnerable groups, such as AI/AN, Black, Hispanic, and rural populations. In addition, although abstinence from drug use remains a goal for many people with SUDs, approaches that reduce use, minimize harms, and address related symptoms also improve health and quality of life. NIDA supports research to address drug craving, withdrawal symptoms, comorbid mental illnesses, and poor sleep, as well as research to reduce harms such as overdose and infectious diseases. (See Program Portrait “Reducing Harms Associated with Substance Use.”)
Translation and Implementation of Evidence Based Practices
NIDA prioritizes research to translate and implement evidenced-based practices to prevent and treat SUD and overdose in diverse settings. The Clinical Trials Network (CTN), expanded in 2020 through funds from the HEAL Initiative®, comprises 16 nodes across the country that conduct multi-site clinical trials on new SUD interventions. NIDA supports CTN studies to optimize current treatments for OUD, develop new treatments for OUD and stimulant use disorders, and integrate OUD screening and treatment into emergency departments, hospitals, primary care clinics, and AI/AN communities. A recent CTN study showed that high-dose buprenorphine induction in emergency departments was safe and well-tolerated in people with OUD experiencing withdrawal symptoms, making this a potentially promising approach to help people transition to outpatient treatment.11 Through the Justice Community Opioid Innovation Network (JCOIN) funded by the HEAL Initiative®, NIDA is testing strategies in 36 states to expand effective OUD treatment in justice settings. JCOIN research has shown that providing medications for OUD during incarceration reduces emergency costs12 and could reduce overdose deaths by 30 percent.13 A JCOIN pilot study showed that one-month extended release buprenorphine resulted in greater retention in treatment than sublingual buprenorphine. JCOIN has added research on the impacts of COVID-19 on substance use, COVID-19 testing protocols and vaccinations, and COVID-19-related structural, practice, and policy changes. The Healing Communities Study (HCS) also funded through the HEAL Initiative® aims to reduce opioid-related overdose deaths by 40 percent during the study period in 67 communities heavily impacted by the opioid crisis. The HCS launched community advisory coalitions and data dashboards to guide community-decision making, and communications campaigns to increase awareness of evidence-based practices and reduce stigma against people with OUD. HCS researchers also provided virtual solutions such as virtual naloxone training.
Program Descriptions
Division of Neuroscience and Behavior
NIDA’s Division of Neuroscience and Behavior (DNB) advances knowledge of the basic biological mechanisms that underlie substance use and SUDs and that guide the development of novel prevention and treatment strategies for SUDs and overdose. This includes identifying the effects of illicit substances on brain structure and function throughout the lifespan and across stages of drug use and SUDs. Areas of focus include identifying: genetic variants and epigenetic modifications that determine vulnerability to SUDs; the effects of drugs on gene expression and brain development and function; the nature and dynamics of drug-receptor interactions at the atomic level; and the cellular signaling engaged by these interactions that may underlie the development of addiction. DNB-supported research has elucidated the neurobiology of opioid, nicotinic, cannabinoid, and benzodiazepine receptors, and this knowledge is being leveraged to guide the development of novel therapeutics to treat SUD, the adverse consequences of illicit drugs, and pain.
Reducing Harms Associated with Substance Use
Many harms associated with drug use stem from scarcity of sterile injection equipment, unprotected sex due to drug impairment or as part of transactional sex, and adulteration of drugs with fentanyl. Data show that overdose deaths are significantly reduced in communities that distribute naloxone, and infectious disease transmission is reduced through syringe-services programs (SSP). Yet, these harm reduction strategies are not widely adopted. NIDA supports research to expand use of existing harm reduction services and to test novel approaches.
NIDA supports extensive research on the impact of SSPs, including expanded provision of sterile syringes, HIV testing and linkage to care, integrated PrEP delivery and antiretroviral therapy to reduce HIV transmission, and naloxone distribution to prevent overdose. Novel research is testing models to initiate buprenorphine maintenance treatment in SSPs, implement SSPs in rural communities vulnerable to opioid injection-related HIV outbreaks, and a recently launched multi-site clinical trial is examining whether integrated health services delivered through mobile clinics improves outcomes for both HIV and SUDs. Still other studies are examining the impact of harm reduction policies in the United States and other countries and on public health.
Overdose prevention centers (OPC) aim to provide a space for people to consume pre-obtained drugs in a controlled setting under supervision of trained staff, but data on their effectiveness is limited. NIDA is currently supporting a study at an existing OPC in Vancouver to test its impact on reducing opioid-related harms.
NIDA also supports research on fentanyl countermeasures including fentanyl test strips (FTS) to help people detect fentanyl in drugs and take steps to prevent overdose. Current projects are validating FTS, determining if FTS use results in decreased drug use and increased treatment retention, and testing whether adding FTS education and distribution to overdose counseling programs decreases overdoses.
NIDA will continue to prioritize harm reduction approaches which are vital to national and community efforts to address the addiction and overdose crisis.
The DNB portfolio also includes research that is advancing our understanding of the mechanisms by which neuromodulation, such as transcranial stimulation, deep brain stimulation, and neurofeedback, can be used to treat SUD by identifying specific brain circuits that can be modulated by these approaches with precision to yield therapeutically beneficial effects. DNB-supported research using cutting-edge genetics and neuro-engineering approaches to interrogate and modulate populations of brain cells is revealing a complex map of neural circuits that are engaged by addictive drugs and that underlie their rewarding and aversive effects. Research using advanced computational approaches including theoretical modeling and novel methods for analyzing large, diverse data sets are being used to link SUD-related behaviors to underlying neural mechanisms.
DNB is also pioneering Big Data Science as a tool to understand the biology of addiction. A cross-cutting research theme in the Division is that of sex differences. DNB promotes research to elucidate the neurobiological basis of sex differences in drug effects and in the development of addiction. This is critical for developing individually-tailored prevention and treatment strategies.
Finally, DNB supports a robust research portfolio focused on the shared biological mechanisms underlying drugs and HIV, and how these mechanisms are involved in HIV-associated neurological disorders.
Budget Policy: The FY 2023 President’s Budget request is $603.5 million, an increase of $107.6 million or 21.7 percent compared with the FY 2022 CR level.
Division of Epidemiology, Services, and Prevention Research
NIDA’s Division of Epidemiology, Services, and Prevention Research (DESPR) supports integrated approaches to understanding and addressing the interactions between individuals and environments that contribute to drug use, addiction, and related health problems. Through the annual Monitoring the Future survey of substance use and related attitudes among youth and young adults, the Population Assessment of Tobacco and Health, which collects biospecimens and behavioral data associated with tobacco use, as well as other studies, DESPR monitors trends in drug use, including marijuana, vaping/e-cigarettes, and other drugs, as well as the potential risks and health outcomes related to these behaviors.
Immunotherapies for Substance Use Disorders and Overdose
Immunotherapies, including vaccines and antibody treatments, are an exciting area in NIDA’s medications development research portfolio. Immunotherapies rely on antibodies tailored to bind to a specific drug when it enters the bloodstream, which prevents the drug from entering the brain, therefore decreasing overdose risk and reducing the drug’s rewarding effects. Immunotherapies could be an important treatment tool to prevent relapse for patients with SUDs.
NIDA-funded research on immunotherapies for stimulant use disorders led to the development of IXT-m200, a monoclonal antibody that targets methamphetamine, which has received Fast Track designation from the FDA and is now being studied in emergency department settings in people with methamphetamine overdose. It is the first novel, investigational treatment for methamphetamine addiction ever to advance in the medication development process to a Phase 2 clinical trial. Other NIDA research is currently investigating potential vaccines and monoclonal antibody treatments that target cocaine.
Overdose mortality reached a record high in 2020, with most deaths involving the highly potent synthetic opioid fentanyl, indicating that research on treatments to prevent overdose from fentanyl is more important than ever. The opioid antagonist naloxone is effective at reversing overdose, but it is broken down quickly in the body, requiring repeated dosing to prevent lethal overdoses. An effective immunotherapy targeting fentanyl could potentially provide longer-lasting, preemptive protection from overdose. Through HEAL Initiative® funding, researchers are actively working on developing vaccines and antibody treatments that target fentanyl and other opioids for OUD treatment. One project has led to the first-ever Phase 1 clinical trial of a vaccine targeted against oxycodone, a commonly misused prescription opioid. Researchers are also developing oxycodone and fentanyl vaccines using novel nanovaccine technology.
Continued research on immunotherapies for OUD and other substance use disorders is critical to reducing overdose deaths and developing a broad range of effective treatments for people with SUD.
Preventing the initiation of substance use to minimize risks of harmful consequences is an essential part NIDA’s mission. To this end, DESPR funds a portfolio of prevention research to understand and intervene upon mechanisms that underlie risk for and resilience to drug use and addiction, and common comorbidities. This includes studies on how biological, psychosocial, and environmental factors operate to enhance or mitigate an individual’s propensity to initiate substance use or to escalate from use to misuse to SUD across different developmental stages. This information, along with rapidly growing knowledge about substance use and addiction, is helping to inform the development of evidence-based prevention strategies.
DESPR also supports research on integrating prevention and treatment services into healthcare and community systems to reduce the burden of drug problems across the lifespan. For example, ongoing research is examining efforts to implement evidence-based SUD treatment in jails and prisons, expand the use of effective medications for OUD in primary care settings, develop strategies to reduce transmission of viral infections related to substance use (e.g., HIV and Hepatitis C), and increase uptake and retention in treatment for SUDs and HIV. DESPR also funds research into the efficacy of screening, brief intervention, and referral to treatment in primary care settings for reducing drug use and SUDs.
Budget Policy: The FY 2023 President’s Budget request is $421.3 million, an increase of $75.1 million or 21.7 percent compared with the FY 2022 CR level.
Division of Therapeutics and Medical Consequences Research
NIDA’s Division of Therapeutics and Medical Consequences (DTMC) supports research to evaluate the safety and efficacy of pharmacological and non-pharmacological interventions to prevent and treat SUDs and drug overdose. This work spans all phases of medical product development including synthesis and preclinical evaluation of potential therapeutics, clinical trial design and execution, and preparing regulatory submissions. Through these investments, NIDA helps to mitigate risks of developing new treatments for SUDs. For example, in collaboration with US WorldMeds, DTMC supported clinical trials on LUCEMYRA™, the first medication targeted specifically to treat the physical symptoms associated with opioid withdrawal,14 which was approved by the FDA in May 2018. NIDA also supports research to identify promising compounds and make them more feasible for pharmaceutical companies to complete costly clinical studies for SUD indications. As part of the HEAL Initiative®, described below, DTMC leads efforts to develop safe and effective new and repurposed medications to prevent and treat OUD and overdose.
NIDA is also prioritizing the development of new treatments for stimulant (i.e., cocaine and methamphetamine) overdose and stimulant use disorders. This portfolio includes developing novel pharmacotherapies, repurposing medications already approved by the FDA for other indications, as well as developing novel vaccines and monoclonal antibodies to treat stimulant use disorders. (See “Immunotherapies for Substance Use Disorder and Overdose.”)
Budget Policy: The FY 2023 President’s Budget request is $136.0 million, an increase of $24.2 million or 21.7 percent compared with the FY 2022 CR level.
Center for Clinical Trials Network
The overarching mission of the NIDA Clinical Trials Network (CTN) is to allow treatment providers, treatment researchers, patients, and NIDA to collaboratively develop, validate, refine, and deliver new treatment options to patients. The CTN comprises 16 research nodes with 30 Node principal investigators affiliated with academic medical centers and large health care networks; 2 research coordinating centers; and more than 240 community-based treatment programs and provider organizations. This unique partnership enables the CTN to conduct studies of behavioral, pharmacological, and integrated treatment interventions in multisite clinical trials to test their effectiveness across a broad range of settings and populations. It also allows the CTN to facilitate the transfer of research results to providers and patients. The network evaluates interventions, implementation strategies, and health system approaches to addressing SUD and co-occurring conditions such as mental illnesses and HIV. Using support from the NIH HEAL Initiative®, the CTN was able to add five new nodes, expanding its geographical reach and capacity to develop and test interventions in diverse populations.
Through the HEAL Initiative®, the CTN has launched several multisite trials examining methods for optimizing the treatment of OUD. One study will examine if rapid transition to extended-release naltrexone following detoxification is more effective than standard detoxification and naltrexone initiation. Another study is underway to evaluate strategies to improve medication treatment retention and strategies to improve outcomes among patients who have achieved stable remission on OUD medications and want to discontinue medication. The CTN is conducting studies to evaluate a collaborative care intervention for preventing progression of opioid misuse to OUD, medications for treating OUD during pregnancy, and strategies for integrating OUD screening and treatment into emergency departments, hospitals, primary care clinics, and AI/AN communities. The network has supported studies to capture important data for research on SUD in electronic health record (EHR) systems in primary care and emergency departments and is currently testing clinical decision support that integrates with EHR systems to help doctors diagnose OUD and provide treatment or refer patients to appropriate care. Complementing the work supported through NIDA’s DTMC, CTN studies are investigating the effectiveness and safety of pharmacotherapies, and transcranial magnetic stimulation, for methamphetamine and cocaine use disorders. A CTN study recently demonstrated that a combination of bupropion and extended-release naltrexone successfully reduced methamphetamine use and cravings in adults with methamphetamine use disorder.
Budget Policy: The FY 2023 President’s Budget request is $48.0 million, an increase of $8.6 million or 21.7 percent compared with the FY 2022 CR level.
Office of Translational Initiatives and Program Innovations
NIDA’s Office of Translational Initiatives and Program Innovations (OTIPI) takes research discoveries in prevention, detection, and treatment of SUDs into candidate health applications for commercialization. OTIPI manages NIDA’s Small Business Innovation Research/Small Business Technology Transfer Programs to advance health applications. It also uses novel fit-for-purpose funding authorities, such as Prizes and Open Competitions, and establishes teaching programs that equip scientists with the competence to translate advances in addiction research into products. Many of these efforts take the form of innovative new technology applications, from mobile apps that help patients find open beds in addiction treatment facilities or connect to support communities, to more sophisticated medical devices. These tools provide or support psychosocial and medication-based treatment, help individuals sustain their recovery from SUDs, and even facilitate prevention. For example, reSET and reSET-O are mobile applications (apps) that deliver cognitive behavioral therapy to people with non-opioid SUDs (reSET) and OUD (reSET-O), and were the first “digital medicines” to receive FDA approval for the treatment of addiction.15 With NIDA-support, another company developed a hospital bassinet pad that delivers gentle, random vibrations to reduce irritability and improve cardiorespiratory function in newborns born dependent on opioids, which received breakthrough device designation from the FDA. In addition, a cloud-based referral tool called OpenBeds was expanded to facilitate patient referrals to addiction treatment facilities.16 OTIPI also helps startups develop technology to help people in recovery. For example, Sober Grid is an app that connects patients with others in recovery and with peer coaches to help them remain drug-free.17 We the Village, Inc. uses telehealth and a social support network to deliver a care model based on community reinforcement and family training.18 Finally, to prevent diversion of drugs, one company developed systems to monitor controlled substances in hospitals, and another developed a tool to detect and report illicit online sales of controlled substances.19,20
Budget Policy: The FY 2023 President’s Budget request is $48.5 million, an increase of $8.6 million or 21.7 percent compared with the FY 2022 CR level.
NIH Helping to End Addition Long-term (HEAL) Initiative®
Through the HEAL Initiative®, NIDA continues to expand support for research to combat opioid misuse and addiction and increase the efficiency of translating research into benefits for people. HEAL Initiative® funds are being used to accelerate the development and availability of novel treatments for OUD and overdose, including developing longer-acting formulations of existing OUD drugs like buprenorphine and methadone, and developing novel immunotherapies including vaccines that could block the effect of opioids in the brain to help people with OUD and decrease overdoses.
Opioid misuse often begins during adolescence and young adulthood, so behavioral interventions and treatment options tailored to this population are crucial to maximize positive outcomes. HEAL Initiative® funds are used to support research to develop and test effective technology-driven, scalable interventions that can prevent opioid misuse and OUD among adolescents and young adults, with a focus on vulnerable populations such as AI/AN and Black communities.
Using HEAL Initiative® funds, NIDA supports research to develop effective implementation strategies for evidence-based interventions, with a focus on high-risk populations. The Justice Community Opioid Innovation Network (JCOIN) is testing strategies for expanding effective OUD treatment and care for people in justice settings in partnership with local and state justice systems and community-based treatment providers. This research will help improve OUD treatment access for vulnerable individuals during incarceration and after release. The HEALing Communities Study, an unprecedented multisite implementation study being conducted in 67 communities across New York, Massachusetts, Kentucky, and Ohio, aims to reduce opioid-related overdose deaths by deploying evidence-based strategies to prevent and treat opioid misuse and OUD. Researchers work with community members and local coalitions to launch intervention activities and communications campaigns, engage at-risk populations, create data dashboards to help guide community decision-making, and develop community action plans to implement specific evidence-based practices to facilitate sustainable, successful solutions tailored to the needs of the local communities.
The HEALthy Brain and Child Development Study is a trans-NIH effort led by NIDA with support from HEAL Initiative® and 19 NIH Institutes and Offices to prospectively examine brain and behavioral development in children from birth to 9-10 years of age. This study is establishing a cohort of pregnant women across a variety of regions and demographics in the United States and will follow their children through the first decade of life to determine how environmental factors, including maternal drug exposure and genetics, influence early brain development and behavioral and clinical outcomes, such as mental illnesses and addiction.
Finally, the HEAL Initiative® is building the Integrative Management of chronic Pain and OUD for Whole Recovery (IMPOWR) network to develop effective treatment interventions for people who experience both chronic pain and OUD. The IMPOWR network consists of clinical research centers that collaborate to develop effective interventions, best models of care for delivery of services, and sustainable implementation strategies for a variety of patients with co-occurring chronic pain and OUD or opioid misuse, with an emphasis on highly vulnerable groups, such as AI/AN, Black, Hispanic, and rural populations.
Budget Policy: The FY 2023 President’s Budget request for the HEAL initiative is $396.9 million, an increase of $135.1 million or 51.6 percent compared with the FY 2022 CR level. Including $8.5 million for Research Management and Support, total NIDA funding in FY 2022 for the HEAL initiative is $405.4 million, an increase of $135.1 million or 50.0 percent compared to the FY 2022 CR level.
Intramural Research Program
NIDA conducts research in high priority areas through its Intramural Research Program (IRP). The IRP portfolio includes research to: 1) elucidate the mechanisms underlying the development of SUDs; 2) evaluate potential new therapies for SUDs, including pharmacological and non-pharmacological interventions; and 3) identify and characterize emerging drugs such as synthetic opioids, stimulants, and cannabinoids.
One example of treatment evaluation at the IRP is a bench-to-bedside project in which IRP investigators are testing a novel compound to treat OUD that activates the same receptors as traditional opioids but has only a subset of their cellular actions. IRP investigators are testing whether the compound reduces self-administration of opioids in animal models and people with OUD, and whether it prevents opioid withdrawal with fewer side effects than medications in current use. If successful, this compound could be a new medication for OUD.
The IRP is also working with the National Center for Advancing Translational Sciences on a dopamine D3 receptor antagonist that could be taken together with opioid pain relievers to reduce the chance of developing OUD. Preliminary animal studies suggest that the compound reduces opioid self-administration and drug-seeking behavior without reducing the pain-relieving effects of opioids. This compound holds promise as an adjunct to opioid treatment for pain and potentially for OUD.
Non-pharmacological addiction treatments are also being developed in NIDA’s IRP. The on-site treatment-research clinic includes efforts to develop a smartphone app that uses machine learning to detect or predict stress, craving, and drug use within hours—and a parallel project to develop content that the app could deliver “just in time.” Because current apps purporting to serve these functions do not meet scientific standards of evidence, IRP is addressing a major gap in mobile health. Using passive measurement and digital phenotyping techniques, the IRP is also developing interventions and big data methodologies to prevent HIV transmission associated with unprotected sex in the context of substance use.
Budget Policy: The FY 2023 President’s Budget request is $106.8 million, an increase of $2.6 million or 2.5 percent above the FY 2022 CR level.
Research Management and Support
Research Management and Support (RMS) activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, dissemination of latest research findings and funding opportunities, and evaluation of NIDA’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. RMS staff at NIDA play leadership roles in helping to coordinate NIDA’s involvement in the NIH HEAL Initiative®, spearheading NIH’s response to the opioid overdose epidemic.
In addition to the infrastructure required to support research and training, NIDA strives to provide evidence-based resources and educational materials about substance use and addiction, including information about timely public health topics such as opioid overdose prevention, marijuana research, use rates and consequences of vaping, synthetic drug trends, and medications for treatment of SUDs, including OUD. To this end, the RMS portfolio incorporates education and outreach activities to inform public health policy and practice with the goal of ensuring that NIDA is the primary trusted source for scientific information on substance use and addiction in English and Spanish. Staff supported by NIDA’s RMS budget coordinate key activities that help to train the next generation of addiction scientists. In addition, NIDA’s RMS portfolio includes the NIDAMED initiative, which is aimed at engaging and educating clinicians in training and in practice in the latest science related to substance use and addiction.
Budget Policy: The FY 2023 President’s Budget request is $82.3 million, an increase of $1.8 million or 2.2 percent compared with the FY 2022 CR level.
References
- The FY 2023 President’s Budget proposes to rename the National Institute on Drug Abuse to the National Institute on Drugs and Addiction.
- The FY 2023 President’s Budget proposes to rename the National Institute on Drug Abuse to the National Institute on Drugs and Addiction.
- https://nida.nih.gov/videos/monitoring-future-results-2020-survey
- news.umich.edu/daily-marijuana-use-among-us-college-students-reaches-new-40-year-high/
- https://wonder.cdc.gov/
- https://www.nature.com/articles/s41380-020-00880-7
- www.tandfonline.com/doi/pdf/10.1080/07418825.2012.761721?needAccess=true
- ajph.aphapublications.org/doi/10.2105/AJPH.2021.306431
- pubmed.ncbi.nlm.nih.gov/33211981/
- https://pubmed.ncbi.nlm.nih.gov/34620728/
- www.nih.gov/news-events/news-releases/emergency-department-administered-high-dose-buprenorphine-may-enhance-opioid-use-disorder-treatment-outcomes
- pubmed.ncbi.nlm.nih.gov/34339247/
- pubmed.ncbi.nlm.nih.gov/32712165/
- https://nida.nih.gov/about-nida/noras-blog/2018/05/nida-supported-science-leads-to-first-fda-approved-medication-opioid-withdrawal
- peartherapeutics.com/products/reset-reset-o/
- apprisshealth.com/solutions/openbeds/
- www.sobergrid.com/
- wethevillage.co/
- invistics.com/
- www.s-3.io/