Learn how companies can get up to $3.4 million to develop innovative technologies that can have an impact on Substance Use Disorders.
The roadmap below will walk you through the overall process and timeline. This includes understanding a company’s eligibility, the proposal submission process, and additional resources that can help you increase your chance of success.
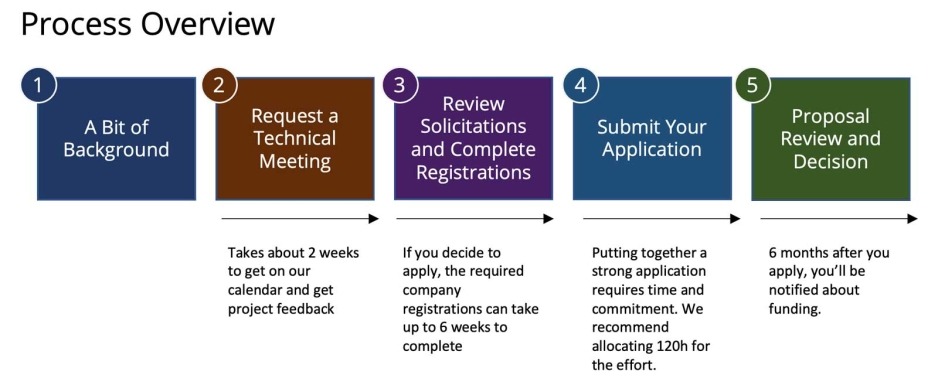
- Text Description of Process Overview graphic
- A Bit of Background
- Request a Technical Meeting - takes about 2 weeks to get on our calendar and get project feedback
- Review Solicitations and Complete Registrations - If you decide to apply, the required company registration can take up to 6 weeks to complete
- Submit your Application - Putting together a strong application requires time and commitment. We recommend allocating 120 hours for the effort
- Proposal Review and Decision - 6 months after you apply, you'll be notified about funding.
Go to Step 1 - A Bit of Background
Learn about the type of small business programs and areas of research we are interested in funding.