Continuation of the PATH Study
NIH’s National Institute on Drug Abuse (NIDA) and FDA’s Center for Tobacco Products (CTP) announced in 2024 the continuation of the Population Assessment of Tobacco and Health (PATH) Study, through the award of a contract to Westat. The continuation of the PATH Study, through this third contract, provides further opportunities for researchers to use study data to advance knowledge about tobacco and health. Since the launch of Wave 1 in 2013, the PATH Study has been in the field continually, conducting 7 main waves of data collection (Waves 1-7), as well as 3 additional special data collections (Waves 4.5, 5.5/Adult Telephone Survey, and 7.5). Wave 8 data collection is currently underway, and this new contract supports at least four additional waves of main data collection, as well as other special data collections.
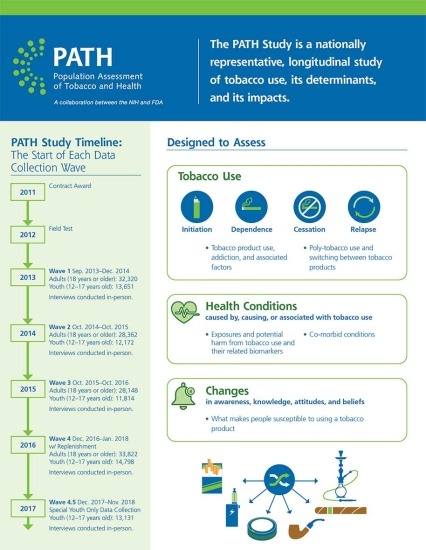
The Population Assessment of Tobacco and Health (PATH) Study is a collaboration between the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), and the Center for Tobacco Products (CTP), Food and Drug Administration (FDA). It was launched in 2011 to inform FDA’s regulatory activities under the Family Smoking and Prevention and Tobacco Control Act. The PATH Study is an ongoing longitudinal cohort study on tobacco use behavior, attitudes and beliefs, and tobacco-related health outcomes. Data collection is planned through 2024.
Interested in Learning More?
Goals:
By monitoring and assessing behaviors, attitudes, biomarkers, and health outcomes associated with tobacco use in the United States, the PATH Study helps enhance the evidence base informing FDA’s regulatory activities related to tobacco. Specifically, the study aims to:
- Examine susceptibility to tobacco product use;
- Study the progression of tobacco product use, including initiation, dependence, cessation and relapse;
- Evaluate patters of tobacco use, including:
- Use of newer products, such as e-cigarettes or ENDS (electronic nicotine delivery systems);
- Poly-use
- Switching products;
- Track potential behavioral and health impacts, including biomarkers of exposure and potential harm; and
- Assess differences in tobacco-related attitudes, behaviors, and health conditions among different subgroups over time.
Findings
About 46,000 people aged 12 years and older, including people who did and did not use tobacco, were included in the first wave of the PATH Study. At the fourth wave in 2017, about 9,800 more participants joined the study.
Initial data on adult and youth tobacco use, published January 2017 in the New England Journal of Medicine, showed that more than 25 percent of American adults were currently using tobacco in 2013-14 and roughly 9 percent of youth reported using tobacco in the past 30 days. Multiple product use was common among people who used tobacco, accounting for roughly 40 percent of adult and youth who used tobacco, with cigarettes and e-cigarettes being the most common combination among both age groups.
Study Team
Scientists at NIDA and the FDA are leading the PATH Study. They are working with Westat, a research company with expertise in survey design, questionnaire development, data collection, and analysis. Westat also manages a team of partner organizations working on the Study.
The Principal Investigator of the PATH Study is located at Roswell Park Comprehensive Cancer Center. Other participating scientists are from:
- Centers for Disease Control and Prevention
- Dartmouth College
- The Medical University of South Carolina
- New York University
- Rutgers University
- Truth Initiative (formerly Legacy)
- The University of California, San Diego
- The University of Minnesota
- The University of Nevada, Reno
- The University of Vermont
- The University of Waterloo
Questions about the PATH Study
- PATH Study FAQs developed by NAHDAP and frequently updated.
- Questions about the collection, content, weighting, documentation, or structure of PATH Study data may be submitted to Westat by e-mail: PATHDataUserQuestions@Westat.com.*
- For specific inquiries regarding the PATH Study BAP and procedures for access to the biospecimens, contact the PATH Study team at PATHStudyBiospecimens@westat.com or review the following FAQs developed by NAHDAP.
- BAP-specific FAQs