Research Highlight
Conformational dynamics of the µ-opioid receptor determine ligand intrinsic efficacy
Jiawei Zhao, Matthias Elgeti, Evan S. O’Brien, Cecília P. Sár, Amal EI Daibani, Jie Heng, Xiaoou Sun, Elizabeth White, Tao Che, Wayne L. Hubbell, Brian K. Kobilka, Chunlai Chen. Nature 629, 474-480 (2024).
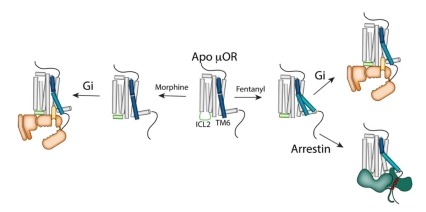
Structurally diverse ligands including alkaloids from the opium poppy and kratom leaf as well as synthetic compounds such as fentanyl bind and activate the μ opioid receptor (μOR). These agonists range in efficacy from full agonists (fentanyl) to strong partial agonists (morphine and mitragynine pseudoindoxyl) to weak partial agonists (buprenorphine). Despite the recent success in the determination of the active-state cryoEM structures of the µOR complexes, the structural and mechanistic basis for differences in ligand efficacies has remained elusive. In a recent effort, scientists used double electron-electron resonance (DEER) and single-molecule fluorescence resonance energy transfer (smFRET) to monitor conformational changes in transmembrane domain 6 (TM6) and the second intracellular loop (ICL2) in response to a panel of µOR ligands with different efficacy profiles. Results from this study showed that full agonists stabilized two distinct outward conformations in TM6, as well as conformational changes in ICL2. The G protein Gi appears to prefer the smaller outward movement of TM6 while arrestin prefers the larger conformational change. The binding of partial agonists such as morphine induce conformational changes in ICL2 without inducing any outward movement of TM6 until the µOR is coupled to Gi. Moreover, the binding of a full agonist induces a conformational change that dramatically lowers GDP affinity within the ternary complex. Findings from this study suggest targeting the sparsely populated conformational states of µOR as a potential approach for the design of therapeutic agents with fewer adverse effects.
Past Highlights
- Structural insights into opposing actions of neurosteroids on GABAA receptors
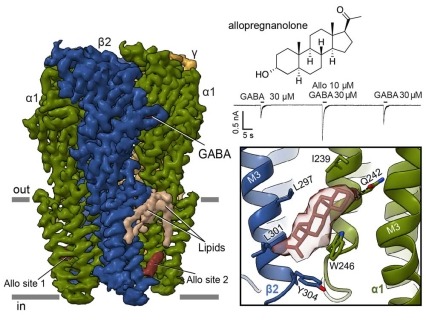
Figure depicting the GABAA cryo-EM structure on the left, bound to both the neurotransmitter GABA, and allopregnanolone, an endogenous positive allosteric modulator. An electrophysiological recording below the chemical structure illustrates the large potentiation of the GABA response when allopregnanolone is co-applied. The structural detail shows the neurosteroid allopregnanolone in its binding site. Structural insights into opposing actions of neurosteroids on GABAA receptors
Dagimhiwat H. Legesse, Chen Fan, Jinfeng Teng, Yuxuan Zhuang, Rebecca J. Howard, Colleen M. Noviello, Erik Lindahl & Ryan E. Hibbs. Nature Communications. 2023 14(1):5091.
Neurosteroids are endogenous modulators of neurotransmission and are emerging as valuable therapeutics for treating depression. In a recent effort, scientists determined how different classes of neurosteroids bind to the GABAA receptor that is the target of the primary inhibitory neurotransmitter of the brain, GABA. This receptor is part of a superfamily of pentameric ligand-gated ion channels. Cryo-electron microscopy revealed the binding site for the positive modulator, allopregnanolone, deep in the membrane, at an interface of two subunits in the receptor (see Figure). This neurosteroid is currently used to treat post-partum depression. The investigators further found that two sulfated steroids, DHEAS and pregnenolone sulfate, bind in the ion channel itself, and thereby inhibit channel activity. The findings reveal how chemically similar modulators act through distinct receptor sites to generate opposing activities. The details of allopregnanolone binding in particular may be helpful in developing improved antidepressants.
- Structure-based design of bitopic ligands for the µ-opioid receptor
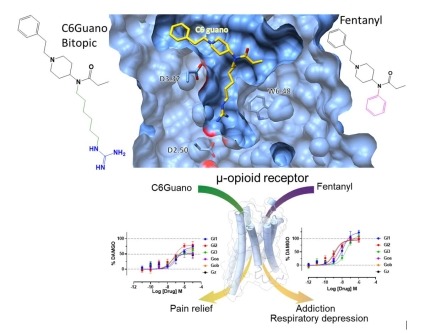
Figure depicting the binding of the bitopic ligand C6Guano engaging the orthosteric ligand binding site and the sodium binding site at the µ-opioid receptor (top) and the differential G-protein coupling efficacy that contributes to differential pharmacological outcomes (bottom). Structure-based design of bitopic ligands for the µ-opioid receptor
Abdelfattah Faouzi, Haoqing Wang, Saheem A. Zaidi, Jeffrey F. DiBerto, Tao Che, Qianhui Qu, Michael J. Robertson, Manish K. Madasu, Amal El Daibani, Balazs R. Varga, Tiffany Zhang, Claudia Ruiz, Shan Liu, Jin Xu, Kevin Appourchaux, Samuel T. Slocum, Shainnel O. Eans, Michael D. Cameron, Ream Al-Hasani, Ying Xian Pan, Bryan L. Roth, Jay P. McLaughlin, Georgios Skiniotis, Vsevolod Katritch, Brian K. Kobilka & Susruta Majumdar. Nature. 2023, 613, 767-774.
Mu-opioid receptor agonists such as fentanyl have long been used for pain management, but are considered a major public health concern due to their adverse side effects, including lethal overdose. In a recent effort, scientists have designed novel bitopic ligands that simultaneously engage the orthosteric binding site and a conserved allosteric sodium ion-binding site within the µ-opioid receptor. Cryo-electron microscopy structure of the µ-opioid receptor bound to the bitopic ligand “C6guano” revealed that the positively charged guanidine group of the ligand engages the Asp2.50 residue in the sodium binding site. The bitopic ligand also shows a distinct G-protein signaling profile compared to fentanyl. In mice, C6guano produced potent antinociception with attenuated adverse effects including reduced respiratory depression. These findings reveal that both efficacy and functional selectivity can be modulated by the design of ligands that occupy the orthosteric and sodium binding sites in a bitopic manner, thus providing a novel strategy to develop safer opioid analgesics.
- Insights into distinct signaling profiles of the µOR activated by diverse agonists
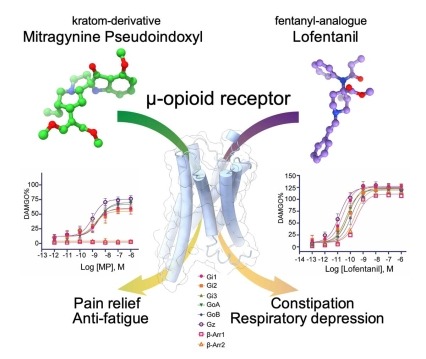
Figure depicting the binding of kratom-derived opioid mitragynine pseudoindoxyl (MP) and fentanyl-derived synthetic opioid lofentanil (LFT) to the mu opioid receptor leading to differences in pharmacological outcomes due to differential engagement of signaling pathways Insights into distinct signaling profiles of the µOR activated by diverse agonists
Qianhui Qu, Weijiao Huang, Deniz Aydin, Joseph M. Paggi, Alpay B. Seven, Haoqing Wang, Soumen Chakraborty, Tao Che, Jeffrey F. DiBerto, Michael J. Robertson, Asuka Inoue, Carl-Mikael Suomivuori, Bryan L. Roth, Susruta Majumdar, Ron O. Dror, Brian K. Kobilka & Georgios Skiniotis. Nat Chem Biol. 2022 Nov 21. doi: 10.1038/s41589-022-01208-y. Epub ahead of print. PMID: 36411392.
Using cryoEM, scientists have now determined the structures of the µ-opioid receptor (MOR) in complex with mitragynine pseudoindoxyl (MP) and lofentanil (LFT). The structures show that the ligands occupy distinct sub-pockets in the same overall binding site within MOR. Atomistic simulations show that the ligands can stabilize different receptor conformations, potentially affecting the propensity of MOR to recruit signaling transducers. These results provide insights into the mechanisms that contribute to low propensity of MP in promoting G protein activation and arrestin recruitment compared to higher efficacy of LFT in signaling through both transducers. These findings reveal molecular mechanisms by which diverse ligands differentially engage distinct signaling pathways while binding to the same orthosteric site. The work was supported by NIDA grants R33DA045884 and R37DA036246.
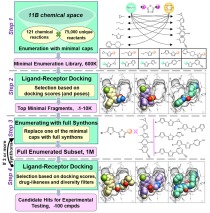
- Synthon-based ligand discovery in virtual libraries of over 11 billion compounds
Illustration of the V-SYNTHES approach to modular screening of Enamine REAL Space. The flowchart on the left shows a general overview of the 4-step algorithm, the panels on the right illustrate examples for each step. Synthon-based ligand discovery in virtual libraries of over 11 billion compounds
Structure-based virtual ligand screening is emerging as a key paradigm for early drug discovery owing to the availability of high-resolution target structures and ultra-large libraries of virtual compounds. In a recent study, NIDA-funded scientists have introduced a conceptually new approach, called V-SYNTHES, that enables computational screening of billions of compounds to identify novel ligands to keep pace with the explosive growth of screening libraries. The method utilizes a hierarchical combinatorial approach that first identifies the best synthon-scaffold combinations as seeds suitable for further growth, and then iteratively elaborates these seeds to select complete molecules with the best docking scores. This method allows rapid detection of the best-scoring compounds in the Giga-scale chemical space while performing docking of only a small fraction (<0.1%) of the library compounds. In this study, the investigators applied the V-SYNTHES approach to screen REAL Space library of more than 11 billion compounds to identify new ligands of the cannabinoid receptors CB1 and CB2. Compared with the standard virtual screening, the V-SYNTHES methods demonstrated dramatically improved hit rate. Follow-up synthesis and evaluations led to the identification of several compounds with sub-micromolar potencies including one ligand with CB2 binding Ki of 0.9 nM that displayed 35 to 65-fold CB2/CB1 functional selectivity as an antagonist.
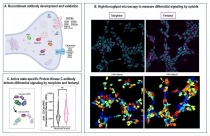
- High-throughput screening and validation of antibodies against synaptic proteins to explore opioid signaling dynamics
(A) Using a novel strategy of antibody development, we generated antibodies to a number of proteins thought to be involved in substance use disorders including receptors, channels and kinases. (B) A high-throughput microscopy/machine learning pipeline was developed for a quick and robust analysis of thousands of cells. (C) An antibody that recognizes the activated form of classical protein kinase C, was used to probe signaling differences between morphine and fentanyl. The analysis revealed significant differences in sustained protein kinase C activation between morphine and fentanyl suggesting a key role for this kinase in opioid receptor signaling and desensitization. Schematic representation created with BioRender.com. Antibodies are powerful tools to explore signal transduction pathways. In a recent effort, NIDA-funded researchers have developed an integrated approach that combines a strategy of antibody development, a time- and cost-effective method of antibody validation, and a high-throughput microscopy/machine learning pipeline to identify antibodies. Using yeast display antibody libraries from the B cells of immunized rabbits and high-throughput functional screening they identified and validated 137 recombinant high affinity antibodies against understudied synaptic proteins. These antibodies could serve as potential tools to shed light on signaling in the central nervous system.
Using a subset of antibodies targeting the opioid receptors they examined the effect of morphine and fentanyl on mu opioid receptor phosphorylation and signaling. Their studies revealed differences in the rate, extent, and site of opioid receptor phosphorylation induced by morphine and fentanyl, thus highlighting the complexity of opioid signaling and illuminating ligand-dependant differential activation and desensitization of mu opioid receptors. These molecular differences may be relevant to the acceleration in opioid overdose deaths that have been attributed to increased use of fentanyl.
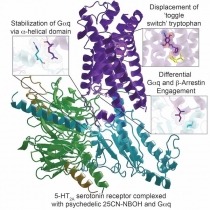
- Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor
-
Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor
Hallucinogens like lysergic acid diethylamide (LSD), psilocybin, and substituted N-benzyl phenylalkylamines are widely used for recreational purposes. In addition to their recreational use, substances such as psilocybin are being considered as therapeutics for many neuropsychiatric disorders including depression, anxiety, and substance abuse. Although the precise mechanisms of action of hallucinogens remain unclear, agonist activity at the 5-HT2A serotonin (5-hydroxytryptamine [5-HT]) receptor is known to play an essential role in their psychedelic effects in humans.
In a landmark study, NIDA-funded scientists have solved active state structure of a prototypical hallucinogen 25-CN-NBOH bound to 5-HT2A receptor in complex with an engineered αq heterotrimer by cryoelectron microscopy (cryo-EM). They also obtained X-ray crystal structures of the arrestin-biased agonist LSD and the inverse agonist methiothepin bound to 5-HT2A receptor. These structures provide insights into the molecular details of both ligand recognition and coupling to the intracellular effectors that are key to gaining a molecular understanding of hallucinogen actions. Moreover, these studies provide a framework for a structure-guided search to identify more selective and efficacious HT2A receptor agonists as potential therapeutic agents for neuropsychiatric disorders.
What We Do:
The Chemistry and Pharmacology Branch (CP) supports research on all aspects of chemistry and pharmacology affected by addictive drugs. The CP Branch maintains, develops, and oversees a portfolio encompassing research, such as:
- Elucidating mechanisms of action, structure-activity relationships, understanding of basic principles involved with biological activity, pharmacology and toxicity,
- Developing new receptor type and subtype specific agents and,
- Supporting research on the pre-clinical development of new pharmacotherapies for the treatment of substance use disorders emphasizing the pre-clinical stages of target identification through hit-to-lead.
Research Interests/Goals:
This branch supports research programs on
- Elucidate effects of drugs on all the physiological systems including the central nervous system and the other physiological systems such as the cardiovascular, pulmonary, and immune.
- Using synthetic, medicinal, Structure-activity studies (SAR) and pharmacological approaches, develops probes for basic research and potential drug leads to treat addictive disorders; Elucidates the chemical mechanism for drug addiction,
- The discovery of endogenous ligands, and the role of endogenous ligands and systems relevant to drug action, and addiction
- The studies of absorption, metabolism, pharmacokinetics, pharmacodynamics, elimination, transport and delivery
- Investigations on analytical methods development, proteomics, protein folding and related functional genomics and structural biology
- Synthesis, structure-function relationships, conformational studies, structural biology, ligand design of all addictive drugs; synthesis of affinity reagents, chemical probes, drug-receptor interaction model to elucidate drug action
- Pharmacological approaches to understand perinatal drug exposure and the effects of confounding factors, such as, maternal and environmental stress, nutrition etc., on neural systems and other organs utero.
Active Funding Opportunities
- Notice of Special Interest (NOSI): Discovery and Development of Natural Products to Treat Substance Use Disorders (SUDs)
NOT-DA-25-032 - Imaging - Science Track Award for Research Transition (I/START)
PAR-24-297 (R03 Clinical Trial Optional) - Chemical Countermeasures Research Program (CCRP) Initiative: Basic Research on The Deleterious Effects of Acute Exposure to Ultra-Potent Synthetic (UPS) Opioids
RFA-DA-26-034 (R01 Clinical Trial Not Allowed) - Notice of Special Interest (NOSI): Development and Application of Novel Chemical Approaches to Discover Therapeutic Targets for Substance Use Disorders
NOT-DA-25-027 - Notice of Special Interest (NOSI): Chemoproteomic Approaches for Discovery of Targets and Therapeutics to Treat Substance Use Disorders
NOT-DA-24-005 (R01, R21, R03, K99/R00, K01, CEBRA, NRSA) - Notice of Special Interest (NOSI) Targeting Epigenetic Regulators for Treating Addiction and Substance Use Disorders
NOT-DA-24-004 (R01, R21, R03, K99/R00, K01, CEBRA, NRSA) - Avenir Award Program for Chemistry and Pharmacology of Substance Use Disorders
RFA-DA-24-007 (DP1 Clinical Trial Not Allowed)
Other Participating Programs
- HEAL Initiative-Early-Stage Discovery of New Pain Targets Within the Understudied Druggable Proteome
PAR-25-154 (R03 Clinical Trial Not Allowed) - Translational Research in Maternal and Pediatric Pharmacology and Therapeutics
PAR-25-110 (R01 Clinical Trial Optional)
PAR-25-111 (R21 Clinical Trial Optional)